References
Addison R, Mould C (2002) Risk assessment in suprapubic catheterisation. Nurs Standard 14(36): 43–6
All Party Parliamentary Group for Continence Care (2013) Continence Services. England. Survey Report. Available online: www.appgcontinence.org.uk/pdfs/Continence%20Care%20Services%20England%20Report%202013.pdf
Bard (2004) Speciality Foley catheters, sales tarining reference guide. Interventional Urology
Charous BL, Blanco C, Tarlo S, et al (2002) Natural rubber latex allergy after 12 years: Recommendations and perspectives. J Allergy Clin Immunol 109(1): 31–4
El Masri WS, Patil S, Prasanna KV, Chowdhury JR (2014) To cystoscope or not to cystoscope patients with traumatic spinal cord injuries managed with indwelling urethral or suprapubic catheters? That is the question! Spinal Cord 52: 49–53
European Association Urology (2012) Catheterisation: indwelling catheters in adults. Available online: www.nursing.nl/PageFiles/11870/001_1391694991387.pdf
Evans A, Godfrey H, Fraczyk L (2001) An audit of problems associated with urinary catheter withdrawal. Br J Community Nurs 6(10): 511–19
Fenelly RCL, Hopely IB, Wells NT (2015) Urinary catheters: history, current status, adverse events and research agenda. J Med Engineering Technol 39(8): 459–70
Ford J, Hughes G, Phillips P (2017) Literature review of silver-coated urinary catheters. Medidex Medical Device Index. Available online: www.medidex.com/research/856-silver-catheter-review-2016-update.html (accessed 11 October, 2017)
Geng V, Cobussen-Boekhorst H, Farrell J, et al (2012) Catheterisation: indwelling catheters in adults. European Association of Urology Nurses, The Netherlands
Gibney LE (2016) Blocked urinary catheters: can they be better managed? Br J Nurs 25(15): 828–33
Johnson JR, Kuskowski MA, Wilt TJ (2006) Systematic review: antimicrobial urinary catheters to prevent catheter-associated urinary tract infection in hospitalized patients. Ann Intern Med 144(2): 116–26
Kleinpell RM, Munro CL, Giuliano KK (2008) Targeting health care–associated infections: evidence-based strategies. In: Hughes RG, ed. Patient Safety and Quality: an evidence-based handbook for nurses. Agency for Healthcare Research and Quality, Rockville, USA: Chap 42
Lawrence EL, Turner I G (2006a) Characterisation of the internal and external surfaces of four types of Foley catheter using SEM and profilometry. J Mater Sci: Mater Med 17: 1421–31
Lawrence EL, Turner IG (2006b) Kink, flow and retention properties of urinary catheters part 1: Conventional foley catheters. J Materials Sci: Materials in Medicine 17: 147–52
Lee S, Kim AW, Cho Y, et al (2004) A comparative multicentre study on the incidence of catheter-associated urinary tract infection between nitrofurazonecoated and silicone catheters. Int J Antimicrob Agents 24S: S65–S69
Mercer Smith J (2003) Indwelling catheter management: from habit-based to evidence-based practice. Ostomy Wound Management 49(12): 34–45
Moore K N, Hunter K F, McGinnis R, et al (2009) Do catheter washouts extend patency time in long-term indwelling urethral catheters? A randomised controlled trial of acidic washout solution, normal saline washout, or standard care. J Wound, Ostomy Continence Nurs 36(1): 82–90
Morris NS, Stickler DJ, Winters C (1997) Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms? Br J Urol 80: 58–63
National Institute for Health and Care Excellence (2012) Healthcare-associated infections: prevention and control in primary and community care. NICE, London. Available online: www.nice.org.uk/guidance/cg139
National Institute for Health and Care Excellence (2015) Urinary incontinence: the management of urinary incontinence in women. NICE, London. Available online: www.nice.org.uk/guidance/qs77
NHS (2009) Rapid Response Report: Minimising risks of suprapubic catheter insertion (adults only). National Patient Safety Agency. Available online: www.nrls.npsa.nhs.uk/resources/patient-safetytopics/medical-device-equipment/?entryi d45=61917&q=0%c2%acsuprapubic+cath eter%c2%ac
Parkin J, Scanlan J, Woolley M, Grover D, Evans A and Fenely RCL (2002) Urinary catheter ‘deflation cuff’ formation: clinical audit and quantitiative in vitro analysis. BJU International 90: 666–71
Pratt RJ, Pellowe CM, Wilson JA, et al (2007) epic2: National evidence-based guideline for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 65 Suppl 1: S1–64
Robinson J (2003a) Suprapubic catheter removal: the cuffing effect of deflated catheter balloons. Br J Community Nurs 8(5): 205–8
Robinson J (2003b) Deflation of a Foley catheter balloon. Nurs Standard 17(27): 33–8
Stickler D, Young R, Jones G, Sabbuba N, Morris N (2003) Why are Foley catheters so vulnerable to encrustation and blockage by crystalline bacterial biofilm? Urol Res 31(5): 306–11
Stickler DJ, Morgan SD (2008) Observations on the development of the crystalline bacterial biofilms that encrust and block Foley catheters. J Hosp Infect 69: 350–60
Tunney MM, Gorman SP (2002) Evaluation of a poly(vinyl pyrollidone)-coated biomaterial for urological use. Biomaterials 23: 4601–08
Verma A, Bhani D, Tomar V, Bacchiwal R, Yadav S (2016) Differences in bacterial colonization and biofilm formation property of uropathogens between the two most commonly used indwelling urinary catheters. J Clin Diagn Res 10(6): PCO1–PCO3
All Party Parliamentary Group for Continence Care (2013) Continence Services. England. Survey Report. Available online: www.appgcontinence.org.uk/pdfs/Continence%20Care%20Services%20England%20Report%202013.pdf
Bard (2004) Speciality Foley catheters, sales tarining reference guide. Interventional Urology
Charous BL, Blanco C, Tarlo S, et al (2002) Natural rubber latex allergy after 12 years: Recommendations and perspectives. J Allergy Clin Immunol 109(1): 31–4
El Masri WS, Patil S, Prasanna KV, Chowdhury JR (2014) To cystoscope or not to cystoscope patients with traumatic spinal cord injuries managed with indwelling urethral or suprapubic catheters? That is the question! Spinal Cord 52: 49–53
European Association Urology (2012) Catheterisation: indwelling catheters in adults. Available online: www.nursing.nl/PageFiles/11870/001_1391694991387.pdf
Evans A, Godfrey H, Fraczyk L (2001) An audit of problems associated with urinary catheter withdrawal. Br J Community Nurs 6(10): 511–19
Fenelly RCL, Hopely IB, Wells NT (2015) Urinary catheters: history, current status, adverse events and research agenda. J Med Engineering Technol 39(8): 459–70
Ford J, Hughes G, Phillips P (2017) Literature review of silver-coated urinary catheters. Medidex Medical Device Index. Available online: www.medidex.com/research/856-silver-catheter-review-2016-update.html (accessed 11 October, 2017)
Geng V, Cobussen-Boekhorst H, Farrell J, et al (2012) Catheterisation: indwelling catheters in adults. European Association of Urology Nurses, The Netherlands
Gibney LE (2016) Blocked urinary catheters: can they be better managed? Br J Nurs 25(15): 828–33
Johnson JR, Kuskowski MA, Wilt TJ (2006) Systematic review: antimicrobial urinary catheters to prevent catheter-associated urinary tract infection in hospitalized patients. Ann Intern Med 144(2): 116–26
Kleinpell RM, Munro CL, Giuliano KK (2008) Targeting health care–associated infections: evidence-based strategies. In: Hughes RG, ed. Patient Safety and Quality: an evidence-based handbook for nurses. Agency for Healthcare Research and Quality, Rockville, USA: Chap 42
Lawrence EL, Turner I G (2006a) Characterisation of the internal and external surfaces of four types of Foley catheter using SEM and profilometry. J Mater Sci: Mater Med 17: 1421–31
Lawrence EL, Turner IG (2006b) Kink, flow and retention properties of urinary catheters part 1: Conventional foley catheters. J Materials Sci: Materials in Medicine 17: 147–52
Lee S, Kim AW, Cho Y, et al (2004) A comparative multicentre study on the incidence of catheter-associated urinary tract infection between nitrofurazonecoated and silicone catheters. Int J Antimicrob Agents 24S: S65–S69
Mercer Smith J (2003) Indwelling catheter management: from habit-based to evidence-based practice. Ostomy Wound Management 49(12): 34–45
Moore K N, Hunter K F, McGinnis R, et al (2009) Do catheter washouts extend patency time in long-term indwelling urethral catheters? A randomised controlled trial of acidic washout solution, normal saline washout, or standard care. J Wound, Ostomy Continence Nurs 36(1): 82–90
Morris NS, Stickler DJ, Winters C (1997) Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms? Br J Urol 80: 58–63
National Institute for Health and Care Excellence (2012) Healthcare-associated infections: prevention and control in primary and community care. NICE, London. Available online: www.nice.org.uk/guidance/cg139
National Institute for Health and Care Excellence (2015) Urinary incontinence: the management of urinary incontinence in women. NICE, London. Available online: www.nice.org.uk/guidance/qs77
NHS (2009) Rapid Response Report: Minimising risks of suprapubic catheter insertion (adults only). National Patient Safety Agency. Available online: www.nrls.npsa.nhs.uk/resources/patient-safetytopics/medical-device-equipment/?entryi d45=61917&q=0%c2%acsuprapubic+cath eter%c2%ac
Parkin J, Scanlan J, Woolley M, Grover D, Evans A and Fenely RCL (2002) Urinary catheter ‘deflation cuff’ formation: clinical audit and quantitiative in vitro analysis. BJU International 90: 666–71
Pratt RJ, Pellowe CM, Wilson JA, et al (2007) epic2: National evidence-based guideline for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 65 Suppl 1: S1–64
Robinson J (2003a) Suprapubic catheter removal: the cuffing effect of deflated catheter balloons. Br J Community Nurs 8(5): 205–8
Robinson J (2003b) Deflation of a Foley catheter balloon. Nurs Standard 17(27): 33–8
Stickler D, Young R, Jones G, Sabbuba N, Morris N (2003) Why are Foley catheters so vulnerable to encrustation and blockage by crystalline bacterial biofilm? Urol Res 31(5): 306–11
Stickler DJ, Morgan SD (2008) Observations on the development of the crystalline bacterial biofilms that encrust and block Foley catheters. J Hosp Infect 69: 350–60
Tunney MM, Gorman SP (2002) Evaluation of a poly(vinyl pyrollidone)-coated biomaterial for urological use. Biomaterials 23: 4601–08
Verma A, Bhani D, Tomar V, Bacchiwal R, Yadav S (2016) Differences in bacterial colonization and biofilm formation property of uropathogens between the two most commonly used indwelling urinary catheters. J Clin Diagn Res 10(6): PCO1–PCO3



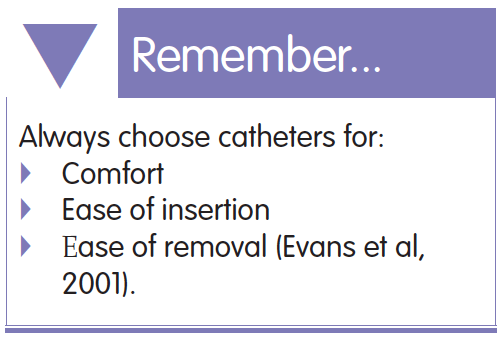 The Foley indwelling catheter is the most common design of catheter generally used for both urethral and suprapubic catheterisation. It was first described by Frederick Foley in the 1930s and since then very little has changed in the design. However, what has changed are the materials used to manufacture the catheters (Fenelly et al, 2015). These continue to evolve in the quest to find the ideal catheter, which will not cause the user problems or at least minimise them. Healthcare professionals should be aware of the different types of catheter available and make sure that they select the best catheter for each individual patient. There is a huge choice of catheters available on the market nowadays, but looking at some of the evidence available can help with making an informed choice.
The Foley indwelling catheter is the most common design of catheter generally used for both urethral and suprapubic catheterisation. It was first described by Frederick Foley in the 1930s and since then very little has changed in the design. However, what has changed are the materials used to manufacture the catheters (Fenelly et al, 2015). These continue to evolve in the quest to find the ideal catheter, which will not cause the user problems or at least minimise them. Healthcare professionals should be aware of the different types of catheter available and make sure that they select the best catheter for each individual patient. There is a huge choice of catheters available on the market nowadays, but looking at some of the evidence available can help with making an informed choice. The uneven surfaces of catheters allow bacteria in the urine to colonise catheters and form biofilms on both the internal and external catheter surface (Tunney and Gorman, 2002; Stickler et al, 2003). Studies have shown that silicone catheters are the smoothest when examined under electron microscopes. However, all catheters, including silicone ones, have cracks and uneven surfaces, which is down to the manufacturing process and the bonding of the catheter coatings to the latex base (Lawrence and Turner, 2006a). Bacteria attach themselves in these areas and rapidly multiply forming a thick matrix known as a biofilm. Once within the biofilm, bacteria are protected and very resistant to antibiotics or catheter maintenance solutions (Gibney, 2016).
The uneven surfaces of catheters allow bacteria in the urine to colonise catheters and form biofilms on both the internal and external catheter surface (Tunney and Gorman, 2002; Stickler et al, 2003). Studies have shown that silicone catheters are the smoothest when examined under electron microscopes. However, all catheters, including silicone ones, have cracks and uneven surfaces, which is down to the manufacturing process and the bonding of the catheter coatings to the latex base (Lawrence and Turner, 2006a). Bacteria attach themselves in these areas and rapidly multiply forming a thick matrix known as a biofilm. Once within the biofilm, bacteria are protected and very resistant to antibiotics or catheter maintenance solutions (Gibney, 2016).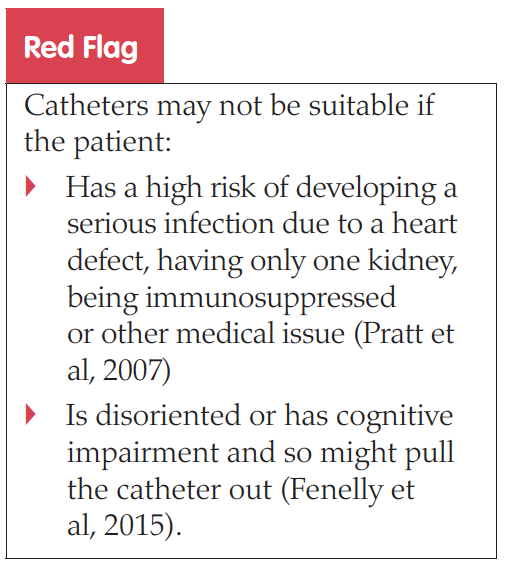 For some patients, long-term suprapubic catheterisation may be the preferable option. It is seen as having fewer complications than urethral catheterisation and allows the patient more dignity, comfort and convenience when it comes to caring and changing the catheter (NHS, 2009). However, as previously said, they are not problem-free and complications such as UTIs, urine leakage around the abdominal catheter site or from the urethra, and bladder spasms are common (Addison and Mould, 2002). They are also prone to infection around the catheter site and granulation of tissue in this area, which can lead to pain and difficulty with catheter removal. This is not helped by catheter balloon cuffing.
For some patients, long-term suprapubic catheterisation may be the preferable option. It is seen as having fewer complications than urethral catheterisation and allows the patient more dignity, comfort and convenience when it comes to caring and changing the catheter (NHS, 2009). However, as previously said, they are not problem-free and complications such as UTIs, urine leakage around the abdominal catheter site or from the urethra, and bladder spasms are common (Addison and Mould, 2002). They are also prone to infection around the catheter site and granulation of tissue in this area, which can lead to pain and difficulty with catheter removal. This is not helped by catheter balloon cuffing. Besides choosing the best catheter for the job, healthcare professionals should also educate the patient and carers on catheter care and personal hygiene and the importance of good fluid intake to ensure steady flow of urine through the catheters. Good flow has been shown to reduce infection and consequently encrustation and blockage. Catheter maintenance solutions may be used to irrigate the catheter and dissolve the encrustation, which may help prolong the life of the catheter. Some healthcare professionals advocate regular washouts with chemical solutions (e.g. Opitflo® G [Bard]), or normal saline (Gibney, 2016). However, Moore et al (2009) found no significant difference between carrying out regular washouts or not on patients with long-term catheters, and the reality is that their efficacy may differ from patient to patient (Moore et al, 2009).
Besides choosing the best catheter for the job, healthcare professionals should also educate the patient and carers on catheter care and personal hygiene and the importance of good fluid intake to ensure steady flow of urine through the catheters. Good flow has been shown to reduce infection and consequently encrustation and blockage. Catheter maintenance solutions may be used to irrigate the catheter and dissolve the encrustation, which may help prolong the life of the catheter. Some healthcare professionals advocate regular washouts with chemical solutions (e.g. Opitflo® G [Bard]), or normal saline (Gibney, 2016). However, Moore et al (2009) found no significant difference between carrying out regular washouts or not on patients with long-term catheters, and the reality is that their efficacy may differ from patient to patient (Moore et al, 2009).