References
Adams D, Bucior H, Day G, Rimmer JA. (2012) HOUDINI: make that urinary catheter disappear — nurse-led protocol. J Infect Prevent 13(20): 44–6
Addison R (2001) Practical procedures: trial removal of a catheter. Nurs Times 97(4): 14–15
Andreessen L, Wilde MH, Herendeen P (2012) Preventing catheter-associated urinary tract infections in acute care: the bundle approach. J Nurs Care Qual 27(3): 209–17
Ansell T, Harari D (2017) Urinary catheter-related visits to the emergency department and implications for community services. Br J Nurs 26(9 suppl): S4–11
Bardsley A (2017) How to remove an indwelling urinary catheter in female patients. Nurs Standard 31(19): 42–5
Burkhard FC, Lucas MG, Berghmans LC, Bosch JLHR, Cruz F, Lemack GE, Nambiar AK, Nilsson CG, Pickard R, Tubaro A (2016) EAUN Guidelines on Urinary Incontinence in Adults. European Association of Urology Nurses, the Netherlands
Carter NM, Reitmeier L, Goodloe LR (2014) An evidence based approach to the prevention of catheter-associated urinary tract infections. Urologic Nurs 34(5): 238–41
Chapple A, Prinijha S, Feneley R, Ziebland S (2016) Drawing on accounts of longterm urinary catheter use: design for the seemingly mundane. Qual Health Res 26(2): 154–63
Chenoweth C, Saint S (2013) Preventing catheter-associated urinary tract infections in the intensive care unit. Crit Care Clin 29(1): 19–32
Colley W (2015) Use of frequency volume charts and voiding diaries. Nurs Times 11(5): 12–15
Emberton M, Fitzpatrick J (2008) The Reten- World survey of the management of acute urinary retention: preliminary results. BJU Int 101(Suppl 3): 27–32
Feneley R, Hopley I, Wells P (2015) Urinary catheters history: current status, adverse events and research agenda. J Med Eng Technol 39(8): 459–70
Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT (2011) Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheterassociated urinary tract infections in intensive care units. J Nursing Care Qual 26(2): 101–9
Gage H, AveryM, Flannery C, Williams P, Fader M (2017) Community prevalence of long-term urinary catheters use in England. Neurourol Urodyn 36(2): 293–6
Gallien P, Reymann J, Amarenco G, Nicolas B, de Se`ze M, Bellissant E (2005) Placebo controlled randomized double blind study of the effects of botulinum A toxin in detrusor sphincter dyssynergia in multiple sclerosis patients. J Neurol Neurosurg Psychiatry 76: 1670–3
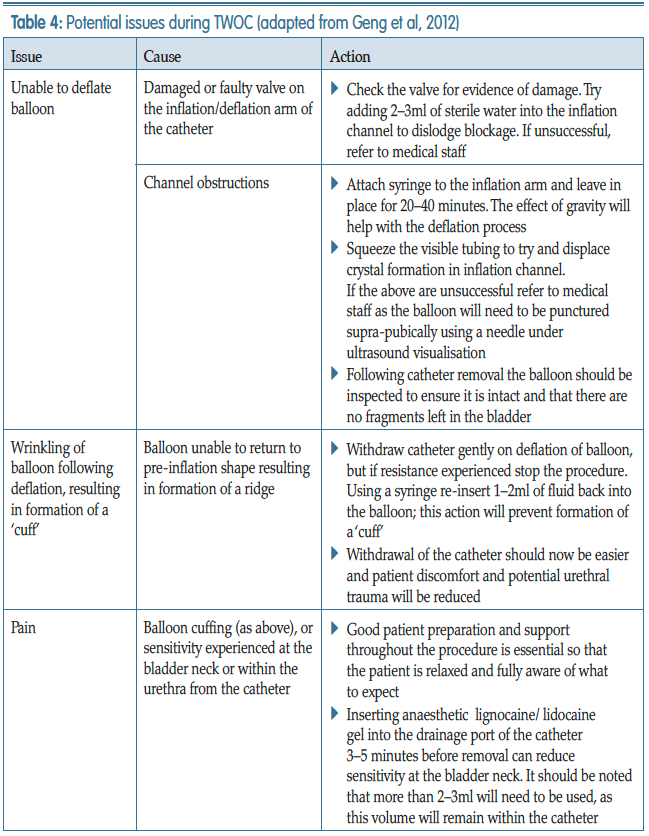
Geng V, Cobussen-Boekhorst H, Farrell J (2012) Catheterisation: indwelling in adults. European Association Of Urology Nurses, The Netherlands
Ghalayini I, Al-Ghazo M, Pickard R (2005) A prospective randomized trial comparing transurethral prostatic resection and clean intermittent self-catheterization in men with chronic urinary retention. BJU Int 96: 93–7
Gilbert R (2006) Procedure to undertake a trial without catheter. Nurs Times 102(42): 48–50
Hanno PM, Burks DA, Clemens JQ (2011) AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol 185(6): 2162–170
Health Protection Scotland (2012) Scottish National Point Prevalence Survey of Healthcare Associated Infection and Antimicrobial Prescribing 2011. Available online: http://bit.ly/1SJCIcK
Holroyd S (2020) Frequency volume charts and fluid balance monitoring: getting it right. J Community Nurs 34(1): 40–3
Kelleher M (2001) Removal of urinary catheters: midnight vs 06.00 hours. Br J Nurs 11(2): 84–90
Kuppusamy S, Gillatt D (2011) Managing patients with acute urinary retention. Practitioner 255(1739): 21–3, 2–3
Loveday HP, Wilson JA, Pratt RJ (2014) EPIC 3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 86(suppl 1): S1–S70
Feneley R, Hopley I, Wells P (2015) Urinary catheter history: current status, adverse events and research agenda. J Med Eng Technol 39(8): 459–70
Mavin C, Mills G (2015) Using quality improvement methods to prevent catheter-associated UTI. Br J Nurs Urology Supplement 24(18): S22–S28
Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S (2014) Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf 23: 277–89
National Institute for Health and Care Excellence (2014) Long-term urinary catheters: prevention and control of healthcare associated infections in primary and community care. NICE, London
NHS Improvement (2017) Preventing healthcare associated Gram-negative bloodstream infections: an improvement resource. Public Health England, London
NHS Improvement (2019) Urinary catheter tools. Available online: https:// improvement.nhs.uk/resources/urinarycatheter- tools/
Newman DK (2007) The indwelling urinary catheter: principles for best practice. J Wound Ostomy Continence Nurs 34(6): 655–61
Purvis S, Gion T, Kennedy G, Rees S, Safdar N, VanDenBergh S, Weber J (2014) Catheter-associated urinary tract infection: a successful prevention effort employing a multipronged initiative at an academic medical center. J Nurs Care Qual 29(3): 141–8
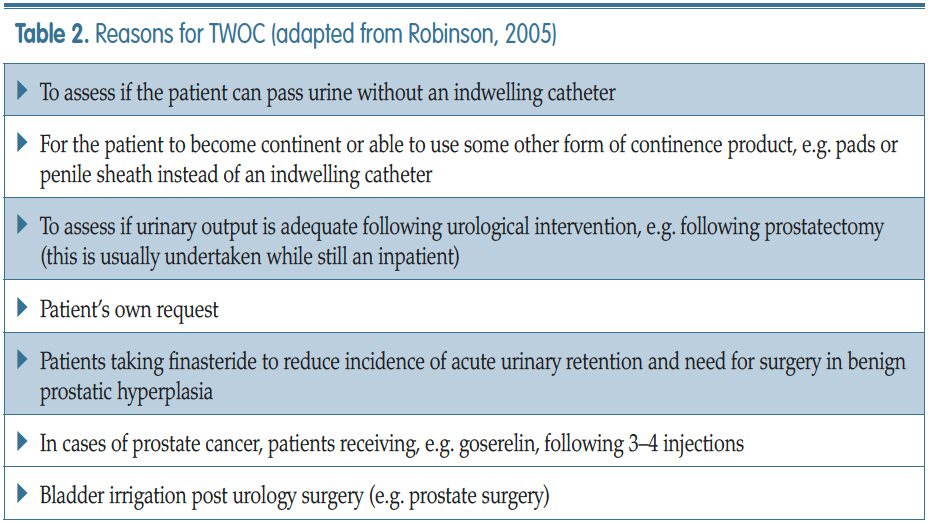
Robinson J (2005) Removing indwelling catheters: trial without catheter in the community. Br J Community Nurs 10(12): 553–4, 556–7
Royal College of Nursing (2019) Catheter Care: RCN Guidance for nurses. RCN London
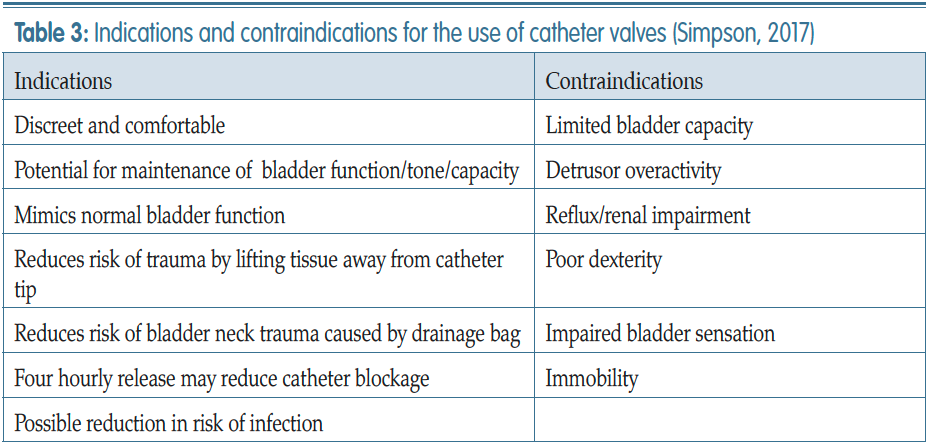
Simpson P (2017) Long-term urethral catheterisation: guidelines for community nurses. Br J Nurs 26(9 suppl): S22–26
Tay I J, Lyons H, Karrouze I, Taylor C, Khan AH, Thompson PM (2016) Impact of the lack of community catheter care services on the emergency department. BJU Int 118(2): 32734
Warering M (2001) Proving a domiciliary urology service. Professional Nurse 12(3): 169–75
Warrilow M, Williams D, Guest J (2004) The introduction of a trial without catheter model in primary care. Br J Nurs 13(7): 1035–40
Woodward S (2013) Catheter valves: a welcome alternative to leg bags. Br J Nurs 22(11): 650, 652–4
Yarde D (2015) Managing indwelling urinary catheters in adults. Nurs Times 111(22): 12–13
Yates A (2016) Indwelling urinary catheterisation: what is best practice? Br J Nurs 25(9) urol suppl: S4–12
Yatim J, Wong K, Ling M, Tan S, Tan K, Hockenberry M (2016) A nurse-driven process for timely removal of urinary catheters. Int J Urol Nurs 10(3): 167– 72
Addison R (2001) Practical procedures: trial removal of a catheter. Nurs Times 97(4): 14–15
Andreessen L, Wilde MH, Herendeen P (2012) Preventing catheter-associated urinary tract infections in acute care: the bundle approach. J Nurs Care Qual 27(3): 209–17
Ansell T, Harari D (2017) Urinary catheter-related visits to the emergency department and implications for community services. Br J Nurs 26(9 suppl): S4–11
Bardsley A (2017) How to remove an indwelling urinary catheter in female patients. Nurs Standard 31(19): 42–5
Burkhard FC, Lucas MG, Berghmans LC, Bosch JLHR, Cruz F, Lemack GE, Nambiar AK, Nilsson CG, Pickard R, Tubaro A (2016) EAUN Guidelines on Urinary Incontinence in Adults. European Association of Urology Nurses, the Netherlands
Carter NM, Reitmeier L, Goodloe LR (2014) An evidence based approach to the prevention of catheter-associated urinary tract infections. Urologic Nurs 34(5): 238–41
Chapple A, Prinijha S, Feneley R, Ziebland S (2016) Drawing on accounts of longterm urinary catheter use: design for the seemingly mundane. Qual Health Res 26(2): 154–63
Chenoweth C, Saint S (2013) Preventing catheter-associated urinary tract infections in the intensive care unit. Crit Care Clin 29(1): 19–32
Colley W (2015) Use of frequency volume charts and voiding diaries. Nurs Times 11(5): 12–15
Emberton M, Fitzpatrick J (2008) The Reten- World survey of the management of acute urinary retention: preliminary results. BJU Int 101(Suppl 3): 27–32
Feneley R, Hopley I, Wells P (2015) Urinary catheters history: current status, adverse events and research agenda. J Med Eng Technol 39(8): 459–70
Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT (2011) Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheterassociated urinary tract infections in intensive care units. J Nursing Care Qual 26(2): 101–9
Gage H, AveryM, Flannery C, Williams P, Fader M (2017) Community prevalence of long-term urinary catheters use in England. Neurourol Urodyn 36(2): 293–6
Gallien P, Reymann J, Amarenco G, Nicolas B, de Se`ze M, Bellissant E (2005) Placebo controlled randomized double blind study of the effects of botulinum A toxin in detrusor sphincter dyssynergia in multiple sclerosis patients. J Neurol Neurosurg Psychiatry 76: 1670–3
Geng V, Cobussen-Boekhorst H, Farrell J (2012) Catheterisation: indwelling in adults. European Association Of Urology Nurses, The Netherlands
Ghalayini I, Al-Ghazo M, Pickard R (2005) A prospective randomized trial comparing transurethral prostatic resection and clean intermittent self-catheterization in men with chronic urinary retention. BJU Int 96: 93–7
Gilbert R (2006) Procedure to undertake a trial without catheter. Nurs Times 102(42): 48–50
Hanno PM, Burks DA, Clemens JQ (2011) AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol 185(6): 2162–170
Health Protection Scotland (2012) Scottish National Point Prevalence Survey of Healthcare Associated Infection and Antimicrobial Prescribing 2011. Available online: http://bit.ly/1SJCIcK
Holroyd S (2020) Frequency volume charts and fluid balance monitoring: getting it right. J Community Nurs 34(1): 40–3
Kelleher M (2001) Removal of urinary catheters: midnight vs 06.00 hours. Br J Nurs 11(2): 84–90
Kuppusamy S, Gillatt D (2011) Managing patients with acute urinary retention. Practitioner 255(1739): 21–3, 2–3
Loveday HP, Wilson JA, Pratt RJ (2014) EPIC 3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 86(suppl 1): S1–S70
Feneley R, Hopley I, Wells P (2015) Urinary catheter history: current status, adverse events and research agenda. J Med Eng Technol 39(8): 459–70
Mavin C, Mills G (2015) Using quality improvement methods to prevent catheter-associated UTI. Br J Nurs Urology Supplement 24(18): S22–S28
Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S (2014) Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf 23: 277–89
National Institute for Health and Care Excellence (2014) Long-term urinary catheters: prevention and control of healthcare associated infections in primary and community care. NICE, London
NHS Improvement (2017) Preventing healthcare associated Gram-negative bloodstream infections: an improvement resource. Public Health England, London
NHS Improvement (2019) Urinary catheter tools. Available online: https:// improvement.nhs.uk/resources/urinarycatheter- tools/
Newman DK (2007) The indwelling urinary catheter: principles for best practice. J Wound Ostomy Continence Nurs 34(6): 655–61
Purvis S, Gion T, Kennedy G, Rees S, Safdar N, VanDenBergh S, Weber J (2014) Catheter-associated urinary tract infection: a successful prevention effort employing a multipronged initiative at an academic medical center. J Nurs Care Qual 29(3): 141–8
Robinson J (2005) Removing indwelling catheters: trial without catheter in the community. Br J Community Nurs 10(12): 553–4, 556–7
Royal College of Nursing (2019) Catheter Care: RCN Guidance for nurses. RCN London
Simpson P (2017) Long-term urethral catheterisation: guidelines for community nurses. Br J Nurs 26(9 suppl): S22–26
Tay I J, Lyons H, Karrouze I, Taylor C, Khan AH, Thompson PM (2016) Impact of the lack of community catheter care services on the emergency department. BJU Int 118(2): 32734
Warering M (2001) Proving a domiciliary urology service. Professional Nurse 12(3): 169–75
Warrilow M, Williams D, Guest J (2004) The introduction of a trial without catheter model in primary care. Br J Nurs 13(7): 1035–40
Woodward S (2013) Catheter valves: a welcome alternative to leg bags. Br J Nurs 22(11): 650, 652–4
Yarde D (2015) Managing indwelling urinary catheters in adults. Nurs Times 111(22): 12–13
Yates A (2016) Indwelling urinary catheterisation: what is best practice? Br J Nurs 25(9) urol suppl: S4–12
Yatim J, Wong K, Ling M, Tan S, Tan K, Hockenberry M (2016) A nurse-driven process for timely removal of urinary catheters. Int J Urol Nurs 10(3): 167– 72