References
Adams D, Bucior H, Day G, et al (2012) HOUDINI: make that urinary catheter disappear — nurse-led protocol. J Infect Prevent 13(2): 44–6
Beattie M, Taylor J (2011) Silver alloy versus uncoated urinary catheters: a systematic review of the literature. J Clin Nurs 20: 2098–2108
Chang R, Todd GM, Chenoweth CE (2011) Epidemiology of hospital-acquired urinary tract related bloodstream infection at a university hospital. Infect Control Hosp Epidemiol 32(11): 1127–9
Chenoweth C, Saint S (2013) Preventing catheter-associated urinary tract infections in the intensive care unit. Crit Care Clin 29(1): 19–32
Davey G (2015) Troubleshooting indwelling catheter problems in the community. J Community Nurs 29(4): 67–74
Elvy J, Colville A (2009) Catheter associated urinary tract infection: what is it, what causes it, and how can we prevent it? J Infect Prevent 10(2): 36–41
Feneley R, Hopley I, Wells P (2015) Urinary catheters history: current status, adverse events and research agenda. J Med Eng Technol 39(8): 459–70
Garcia MM, Gulati S, Liepmann D, Stackhouse GB, Greene K, Stoller ML (2007) Traditional foley drainage systems — do they drain the bladder? J Urol 177: 203–7
Geng V, Cobussen-Boekhorst H, Farrell J (2012) Catheterisation: Indwelling in Adults. European Association of Urology Nurses (EAUN), Holland
Gibney LE (2016) Blocked urinary catheters: can they be better managed? Br J Nurs 25(15): 828–33
Hanchett M (2002) Techniques for stabilizing urinary catheters. Tape may be the oldest method, but it’s not the only one. Am J Nurs 102(3): 44–8
Hayes W (2009) Looking past the silver lining. Materials Management in Healthcare January: 20–3. Available online: matmanmag.com
Health Improvement Scotland (2004) Urinary Catheterisation and Catheter Care. Available online: www. healthcareimprovementscotland.org/previous_resources/best_practice_ statement/urinary_catheterisation__care. aspx
Healthcare Infection Control Practices Advisory Committee (2009) Guideline for Prevention of Catheter-associated Urinary Tract Infection. Available online: www.cdc.gov/infectioncontrol/pdf/guidelines/cauti-guidelines.pdf
Health Protection Scotland (2012) Scottish National Point Prevalence Survey of Healthcare Associated Infection and Antimicrobial Prescribing 2011. Health Protection Scotland (2017) National point prevalence survey of healthcare associated infection and antimicrobial prescribing 2016. Available online: https://tinyurl.com/y4oynld3
Health and Safety Executive (2011) Natural rubber latex sensitisation in health and social care. Available online: www.hse.gov.uk/foi/internalops/sims/pub_serv/071106.htm
Holroyd S (2016) Innovation in catheter securement devices: minimising risk of infection, trauma and pain. Br J Community Nurs 21(5): 256–9
Kyle G (2009) Lubricating gels in urethral catheterisation — the evidence. Continence UK 3(1): 35–7
Leaver R (2017) Understanding long-term catheterisation for effective bladder drainage. J Community Nurs 31(5): 43–8
Levers H (2014) Switching to an antimicrobial solution for skin cleansing before urinary catheterisation. Br J Community Nurs 19(2): 66–71
Loveday HP, Wilson JA, Pratt RJ (2014) Epic3: national evidence-based guidelines for preventing healthcare associated infections in NHS hospitals in England. J Hosp Infect 86(1 Suppl): S1–70
Marinho S, Oliver P, Hughes D (2013) Chlorhexidine allergy policy screening and management of patients with chlorhexidine allergy. University Hospital of South Manchester NHS Foundation Trust
Moka E, Argyura E, Siafaka I, Vadalouca A (2015) Chlorhexidine: hypersensitivity and anaphylactic reactions in the perioperative setting. J Anaesthesiol Clin Pharmacol 31(2): 145–8
NHS Improvement (2019) Preventing Healthcare-associated Gram-negative Bloodstream Infections: An Improvement Resource. Public Health England, London
National Institute for Health and Care Excellence (2012) Infection: prevention and control of healthcare associated infections in primary and community care. NICE London
National Institute for Health and Care Excellence (2014) Long term urinary catheters: prevention and control of healthcare associated infections in primary and community care. NICE London
National Institute for Health and Care Excellence (2017) Healthcare associated infections preventions and control in primary and community care. Clinical guideline 139. NICE London
National Patient Safety Agency (2009) Rapid Response Report: minimising risks of suprapubic catheter insertion (adults only). Available online: www.nrls.npsa.nhs.uk/alerts/?entryid45=61917
Nazarko L (2012) Intermittent selfcatheterisation and catheter fixation. Br J Community Nurs 18(7): 322–5
Pannek J, Vesweber A (2011) Clinical utility of an antimicrobial blocking solution in patients with an indwelling catheter. Aktuelle Urol 42: 51–4
Pellowe C (2009) EPIC 2 Infection prevention guidelines — best practice. Continence UK 3(3): 28–33
Pickard R, Lam T, MacLennan GL (2012) Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short term catheterisation in hospital: a multicentre randomised controlled trial. Lancet 380(9857): 1927–35
Ramakrishnan K, Mold J (2004) Urinary catheters: a review. Internet J Family Practice 3(2). Available online: http://ispub.com/IJFP/3/2/4596
Rowley S, Clare S, Macqueen S, Molyneux R (2010) ANTT v2: an updated practice framework for aseptic technique. Br J Nurs 19(5): S5–11
Royal College of Nursing (2019) Catheter care: RCN guidance for healthcare professionals. RCN, London. Available online: www.rcn.org.uk/professionaldevelopment/publications/PUB-007313
Royal College of Physicians (2005) Report of the National Audit of Continence Care in Older People (65 years and above) in England, Wales and Northern Ireland. RCP, London
Sandle T (2013) Using an antimicrobial skin cleanser before catheterisation. J Community Nurs 27(5): 30–4
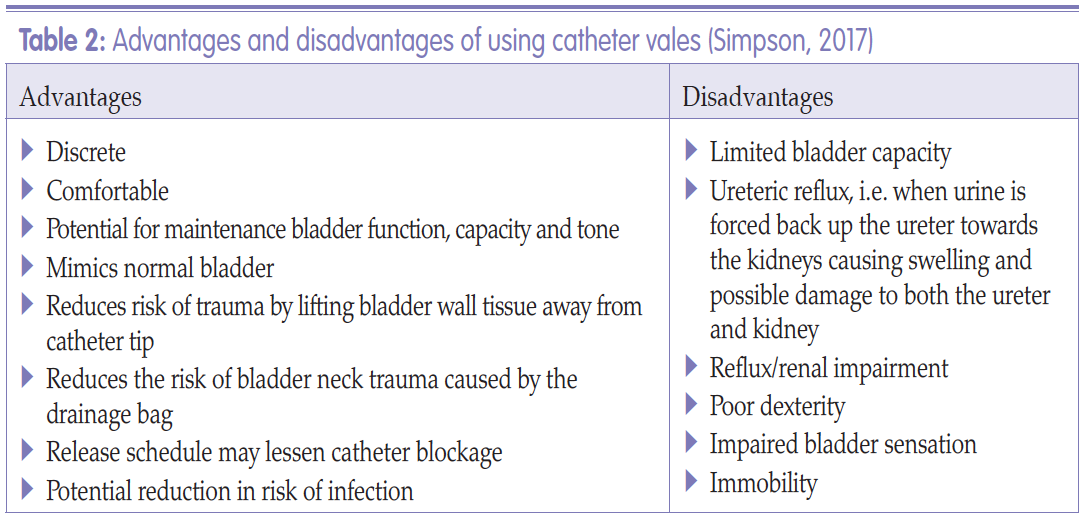
Simpson P (2017) Long-term urethral catheterisation: guidelines for community nurses. Br J Nurs 26(9) urol suppl: S22–26
Sperling H, Eisenhardt A, Mumperow E (2014) Review of the use of triclosan in permanent catheters. Der Urologe 53: 1512–17
Spinks J (2013) Urinary incontinence and the importance of catheter fixation. J Community Nurs 27(5 suppl): 24–9
Stickler DJ, Feneley RCL (2010) The encrustation and blockage of long-term indwelling bladder catheters: a way forward in prevention and control. Spinal Cord 48(11): 784–90
Tenke P, Kovacs B, Bjerklund Johansen TE (2008) European and Asian guidelines on management and prevention of catheter associated urinary tract infections. Int J Antimicrob Agents 31(1 Suppl): S68–78
Turner B, Dickens N (2011) Long-term urethral catheterisation. Nurs Standard 25(24): 49–55
Tzortzis V, Gravas S, Melekos MM, de la Rosette JJ (2009) Intraurethral lubricants: a critical literature review and recommendations. J Endourol 23(5): 821–6
Williams C (2017) Making a choice of catheterisation gel and the role of chlorhexidine. Br J Community Nurs 22(7): 346–51
Wilson M (2016) Urinary catheterisation in the community: exploring challenges and solutions. Br J Community Nurs 21(10): 443
Woodward S (2014) Securing urethral catheters can help to reduce their complications. Br J Neuro Nurs 10(4): 162–5
Wound, Ostomy and Continence Nurses Society (2012) Indwelling urinary catheter securement: best practice for clinicians. Yarde D (2015) Managing indwelling urinary catheters in adults. Nurs Times 111(22): 12–13
Yates A (2016) Indwelling urinary catheterisation: what is best practice? Br J Nurs 25(9) urol suppl: S4–12