Abstract
The human gastrointestinal tract contains the body’s largest community of microbes, comprising trillions of microorganisms collectively referred to as the gut microbiota. The gut microbiota plays a pivotal role in the development, maturation, and differentiation of the immune system and has a significant influence on both the physical and mental development of children, affecting overall health and wellbeing. Numerous factors can affect the diversity of the microbiota, such as mode of delivery in childbirth, environmental exposures, and contact with animals.Disruption or imbalance in the gut microbiota may result in dysbiosis or leaky gut syndrome. Dysbiosis has been linked to a range of diseases in both children and adults, including autism spectrum disorder, attention deficit hyperactivity disorder, asthma, and various allergies. Research indicates that the administration of probiotics may help to restore the balance of the gut microbiota, thereby improving gastrointestinal conditions such as constipation and potentially alleviating certain behavioural symptoms associated with neurodevelopmental disorders.
Introduction
The composition of gut bacteria, known as the ‘microbiota’, plays a crucial role in maintaining the health and wellbeing of children. (Sometimes used interchangeably with the term microbiome, microbiota refers to microorganisms that are found within a specific environment, whereas the microbiome refers to the collection of genomes from all these microorganisms.) There is some debate regarding the timing of bacterial colonisation in the infant gut, with some studies suggesting that this process may begin in utero via the placenta and amniotic fluid. However, it is established that the mode of delivery of a baby can affect the initial bacterial colonisation and the composition of the infant’s microbiome (Bokulich et al, 2016). Infants delivered vaginally typically acquire a microbiota resembling their mother’s vaginal flora, whereas those delivered by caesarean section are more likely to develop a microbiota similar to their mother’s skin flora (Bokulich et al, 2016).Whether a child is breastfed or formula-fed impacts the development of the microbiota. The microbiota of breastfed infants mainly comprises Lactobacillus spp., Staphylococcus spp., and Bifidobacterium spp., whereas the microbiota of formula-fed infants contains Roseburia spp., Clostridium spp., and Anaerostipes spp. (Ronan et al, 2021). When breastfeeding stops, at around 1 year of age, the microbiota evolves to be more adult-like, with microbes that degrade dietary fibres and produce short-chain fatty acids. The microbiota of infants that were formula-fed may mature faster towards an adult composition and include more organisms linked to inflammation than those of breastfed infants (Ihekweazu and Versalovic, 2018).
Research indicates that between the ages of 2–5 years, a child’s gut microbiota typically begins to stabilise into a composition resembling that of an adult (Bokulich et al, 2016; Ihekweazu and Versalovic, 2018). However, other studies suggest that development of the microbiota may continue into puberty or later (Deering et al, 2019). Environmental factors and diet during childhood are considered important influences on the composition of the microbiota. Additional factors, such as having older siblings or exposure to animals and household pets, have also been identified as potential influences on the composition of the microbiota (Milani et al, 2017).
Antibiotic treatment in early life can impact the gut microbiota by reducing bacterial diversity, altering metabolic activity, and enabling antibiotic-resistant microbial strains to establish, which can cause antibiotic-associated diarrhoea (Mukhopadhya et al, 2019). A reduced diversity of microbiota is reported to be associated with an increased risk of issues such as obesity, asthma, and allergies developing later in life (Bokulich et al, 2016).
The gut microbiota is crucial for the body’s immune, metabolic, structural, and neurological functions. The microbes affect gut development, mucosa maturation, differentiation, and immune system. Additionally, the gut microbiota influences both physical and mental health (Adak and Khan, 2019) and plays a vital role in brain development by affecting neurotransmitters like gamma-aminobutyric acid, noradrenaline, dopamine, and serotonin, with more than 90% of serotonin and 50% of dopamine being produced by the gut microbiota.
Serotonin is vital for brain development, and a decrease in levels of serotonin can impair synaptogenesis and brain connectivity, leading to long-term neurodevelopmental issues (Saeed et al, 2022). Most serotonin is produced by the gut microbiota, influencing both mood and gastrointestinal function.
Gut–brain axis and neurodevelopment
The gut microbiota plays a crucial role in various bodily functions and acts very much as an organ within the human body. There is a multidirectional relationship between the microbiota in the gut and the other body systems, forming different systemic axes, including the lung–gut axis, the skin–gut axis and the brain–gut axis (Saeed et al, 2022). An imbalance of the gut microbiota can affect the integrity of any of these axes (Figure 1).Figure 1. Diagram showing the systemic gut axis and how dysbiosis affects health. From Gebrayel et al (2022).
.jpg)
The microbiota–gut–brain axis involves three pathways—endocrine/systemic, immune, and neuronal—which interact and overlap. The microbiota influences various CNS processes and neurodevelopment, including the permeability of the blood–brain barrier, synaptic pruning, neurogenesis, neuronal signalling, and behaviours like sociability, sensory function, memory, learning, and stress (Wang et al, 2023).
One of the primary pathways for gut–brain interaction involves the vagus nerve, which comprises both afferent and efferent neurons and innervates the digestive tract (Bonaz et al, 2018). The signalling from the gut microbiota to the brain via the vagus nerve is well-documented—for example, feeling ‘butterflies’ in the stomach before an important examination. The gut microbiota also produces numerous neurochemical substances such as catecholamines and serotonin. These play a crucial role in maintaining homeostasis in the body and are used by the brain to regulate basic physiological processes and mental functions such as learning.
An imbalance of the gut microbiota, known as dysbiosis, can lead to an alteration of metabolite profiles, thus influencing the enteric nerve CNS communication, brain immune function, CNS inflammation, and function and integrity of the blood–brain barrier (Ronan et al, 2021). Silva et al (2020) suggested that dysbiosis may play a role in the aetiology and development of neurodevelopment disorders and neuropsychiatric diseases.
It is believed that the gut microbiota may regulate behaviours through the production of neuroactive metabolites, suggesting that gut–brain connections could contribute to social and communication disorders such as autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD) (Ronan et al, 2021). Some children with neurodevelopmental disorders seem to experience pain differently to their typically developing peers. It has been proposed that the gut microbiota might influence the central perception of pain by modulating receptor expression (Defaye et al, 2019). For instance, studies on people with irritable bowel syndrome have observed that certain strains of Lactobacillus can alter the expression of mu-opioid and cannabinoid receptors in intestinal epithelial cells, producing effects similar to those of morphine (Kang et al, 2017).
Dysbiosis and ‘leaky gut’
The intestinal mucosa encounters numerous external antigens, including food antigens, food-borne pathogens, and commensal microbes present in the intestinal lumen. Hence, the intestine functions as a barrier tissue where a monolayer of intestinal epithelial cells forms a multilayered physicochemical barrier.This intestinal epithelial barrier maintains biological homeostasis by keeping external antigens out and preventing internal substances from leaking into the gut. This function relies on tight junctions formed by intestinal epithelial cells (Figure 2). Dysbiosis, or altered microbial composition, can disrupt this barrier. Dysfunction of this barrier, particularly the disruption of tight junctions, often leads to enhanced intestinal permeability, a pathological status termed ‘leaky gut syndrome’ (Kinashi and Hase, 2021).
Figure 2. Diagram showing autoimmune responses induced by dysbiosis and leaky gut syndrome. From Kinashi and Hase (2021).
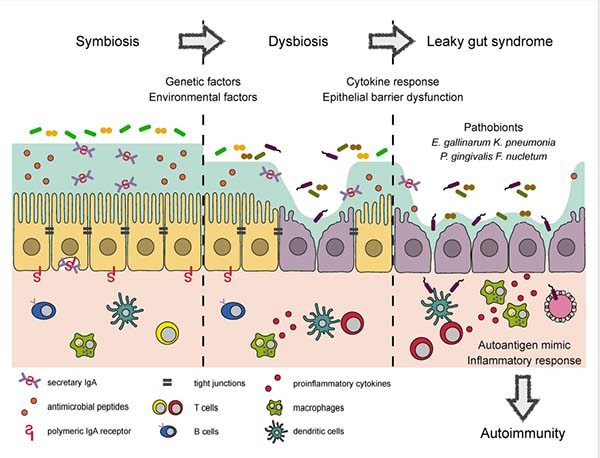
Dysbiosis and leaky gut appear to be connected to the development and/or progression of a number of metabolic and autoimmune systemic diseases, including obesity, non-alcoholic fatty liver disease, neurodegenerative conditions such as Alzheimer’s and Parkinson’s disease, cardiovascular disease, inflammatory bowel disease, and type 1 diabetes mellitus (Di Vincenzo et al, 2024).
Constipation is common among children, affecting up to 28% of all children (Auth et al, 2020) and rising to over 50% among children with Down syndrome and other learning difficulties (Bermudez et al, 2019; Maslen et al, 2020). The composition of the gut microbiota in individuals with constipation differs significantly from those without it, and it is suggested that the microbiota may play a role in the development of functional constipation by influencing peristalsis (Kwiatkowska and Krogulska, 2021). Slow gut transit, which results from low abdominal muscle tone, often contributes to the development of functional constipation in children with Down syndrome. If changes in the microbiota also affect peristalsis, then this could be a factor in both the development of constipation and the limited effectiveness of standard therapy with stool softeners alone.
Management of constipation often requires a multifaceted approach. This includes optimising fluid intake, adjusting diet, and administering appropriate laxative therapy, which may involve a combination of both stool softeners and stimulants. Additional supportive measures include ensuring the correct sitting position is used on a potty or toilet, encouraging physical activity, such as exercises and abdominal massages, as well as ‘cycling’ the legs in infants and pre-mobile toddlers. Given that alterations in gut microbiota can contribute to the pathogenesis of constipation, there is also potential for treatments, such as probiotics, to enhance therapeutic outcomes.
The role that probiotics play in children with neurodevelopmental delays, particularly ASD, have been well documented. Parracho et al (2010) reported fewer ‘hard’ and more ‘formed’ stools in children with ASD treated with probiotic therapy compared those treated with placebo. In a survey of 32 caregivers for ASD children with gastrointestinal distress, West et al (2013) reported that almost half of the respondents (48%) reported decreases in diarrhoea severity and 52% reported decreases in constipation severity following 21 days of probiotic therapy.
A study in Egypt tested a combination of L. acidophilus, Lacticaseibacillus rhamnosus, and B. longum in 30 children with ASD, finding improvements in gastrointestinal symptoms (constipation, stool consistency, flatulence, abdominal pain) and severity of autism, measured by the Autism Treatment Evaluation Checklist (ATEC) (Shaaban et al, 2018). Other research suggests that probiotics may help neurodegenerative disorders. For example, a study of 60 Alzheimer’s patients found that strains from L. acidophilus, Lacticaseibacillus casei, B. bifidum, and L. fermentum improved cognitive function and metabolic levels (Akbari et al, 2016). These are small studies, so larger studies would be required to give more conclusive evidence.
Several studies have examined the impact of probiotics on both gastrointestinal symptoms and behaviour in children with ASD. Guidetti et al (2022) conducted a study in which oral supplementation of a mixed strain probiotic was administered twice daily for 3 months to 61 children with ASD. The results showed significant improvements in communication skills and receptive language as well as general improvement in behaviour.
Santocchi et al (2020) observed improvements in behaviour, including adaptive functioning, developmental pathways and multisensory processing, and gastrointestinal symptoms in children with ASD after taking probiotics. Siemann et al (2017) had previously reported that difficulties in multisensory processing were related to the serotoninergic system, whose levels are influenced by the gut microbiota. Santocchi et al (2020) suggested that probiotics might improve sensory difficulties through the restoration of the serotonin system, which could also lead to reduced gastrointestinal symptoms.
An imbalance in the gut microbiota can lead to dysbiosis and leaky gut, resulting in a range of disorders and chronic health conditions. Exposing children to various environments can promote a healthy gut microbiome. Addressing gastrointestinal issues like constipation enhances gut health. A diverse diet benefits all children, especially those with neurodevelopmental disorders.
Taking probiotics has been shown to improve biodiversity and help balance the gut microbiota, leading to improved gut permeability, cognition, immunity, and preventing dysbiosis. This also reduces gastrointestinal symptoms like inflammation and constipation and may alleviate gastrointestinal and behavioural issues in children with Down syndrome and ASD. However, further research is needed to help establish whether probiotics have a role as a routine component of the mainstream treatment of constipation.
Figure 1 is reproduced from Gebrayel et al (2022) under a Creative Commons Attribution 4.0 International License, and Figure 2 is reproduced from Kinashi and Hase (2021) under a Creative Commons Attribution License (CC BY).
Gastrointestinal problems
Gastrointestinal issues are frequently observed in people with neurodevelopmental disorders, especially among children with ASD, with a reported prevalence of up to 70% (Cao et al, 2013). Research indicates that these issues may be linked to changes in the gut microbiota associated with increased gut permeability (leaky gut), which permits bacterial metabolites to pass through the gut barrier (Sivamaruthi et al, 2020). This process may affect neurodevelopment during early childhood in susceptible individuals via the gut–brain axis.Constipation is common among children, affecting up to 28% of all children (Auth et al, 2020) and rising to over 50% among children with Down syndrome and other learning difficulties (Bermudez et al, 2019; Maslen et al, 2020). The composition of the gut microbiota in individuals with constipation differs significantly from those without it, and it is suggested that the microbiota may play a role in the development of functional constipation by influencing peristalsis (Kwiatkowska and Krogulska, 2021). Slow gut transit, which results from low abdominal muscle tone, often contributes to the development of functional constipation in children with Down syndrome. If changes in the microbiota also affect peristalsis, then this could be a factor in both the development of constipation and the limited effectiveness of standard therapy with stool softeners alone.
Management of constipation often requires a multifaceted approach. This includes optimising fluid intake, adjusting diet, and administering appropriate laxative therapy, which may involve a combination of both stool softeners and stimulants. Additional supportive measures include ensuring the correct sitting position is used on a potty or toilet, encouraging physical activity, such as exercises and abdominal massages, as well as ‘cycling’ the legs in infants and pre-mobile toddlers. Given that alterations in gut microbiota can contribute to the pathogenesis of constipation, there is also potential for treatments, such as probiotics, to enhance therapeutic outcomes.
Probiotics and constipation
Studies have increasingly explored the role of probiotics in managing constipation and other gastrointestinal issues. Several mechanisms may explain their beneficial effects, including the modification of gut microbiota which leads to increased production of lactate and short-chain fatty acids. This alteration reduces the luminal pH, enhancing colonic peristalsis and decreasing gut transit time (Gomes and Morais, 2019).The role that probiotics play in children with neurodevelopmental delays, particularly ASD, have been well documented. Parracho et al (2010) reported fewer ‘hard’ and more ‘formed’ stools in children with ASD treated with probiotic therapy compared those treated with placebo. In a survey of 32 caregivers for ASD children with gastrointestinal distress, West et al (2013) reported that almost half of the respondents (48%) reported decreases in diarrhoea severity and 52% reported decreases in constipation severity following 21 days of probiotic therapy.
A study in Egypt tested a combination of L. acidophilus, Lacticaseibacillus rhamnosus, and B. longum in 30 children with ASD, finding improvements in gastrointestinal symptoms (constipation, stool consistency, flatulence, abdominal pain) and severity of autism, measured by the Autism Treatment Evaluation Checklist (ATEC) (Shaaban et al, 2018). Other research suggests that probiotics may help neurodegenerative disorders. For example, a study of 60 Alzheimer’s patients found that strains from L. acidophilus, Lacticaseibacillus casei, B. bifidum, and L. fermentum improved cognitive function and metabolic levels (Akbari et al, 2016). These are small studies, so larger studies would be required to give more conclusive evidence.
Several studies have examined the impact of probiotics on both gastrointestinal symptoms and behaviour in children with ASD. Guidetti et al (2022) conducted a study in which oral supplementation of a mixed strain probiotic was administered twice daily for 3 months to 61 children with ASD. The results showed significant improvements in communication skills and receptive language as well as general improvement in behaviour.
Santocchi et al (2020) observed improvements in behaviour, including adaptive functioning, developmental pathways and multisensory processing, and gastrointestinal symptoms in children with ASD after taking probiotics. Siemann et al (2017) had previously reported that difficulties in multisensory processing were related to the serotoninergic system, whose levels are influenced by the gut microbiota. Santocchi et al (2020) suggested that probiotics might improve sensory difficulties through the restoration of the serotonin system, which could also lead to reduced gastrointestinal symptoms.
Conclusions
Emerging evidence highlights the significant role of the gut microbiota in the bidirectional communication between the gut and brain, known as the gut–brain axis. This indicates that gut microbes influence neural development, modulate neurotransmission, and affect behaviour. Consequently, they contribute to the pathogenesis and/or progression of various neurodevelopmental, neuropsychiatric, and neurological conditions.An imbalance in the gut microbiota can lead to dysbiosis and leaky gut, resulting in a range of disorders and chronic health conditions. Exposing children to various environments can promote a healthy gut microbiome. Addressing gastrointestinal issues like constipation enhances gut health. A diverse diet benefits all children, especially those with neurodevelopmental disorders.
Taking probiotics has been shown to improve biodiversity and help balance the gut microbiota, leading to improved gut permeability, cognition, immunity, and preventing dysbiosis. This also reduces gastrointestinal symptoms like inflammation and constipation and may alleviate gastrointestinal and behavioural issues in children with Down syndrome and ASD. However, further research is needed to help establish whether probiotics have a role as a routine component of the mainstream treatment of constipation.
Figure 1 is reproduced from Gebrayel et al (2022) under a Creative Commons Attribution 4.0 International License, and Figure 2 is reproduced from Kinashi and Hase (2021) under a Creative Commons Attribution License (CC BY).
References
Adak A, Khan MR (2019) An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 76:473-493. https://doi.10.1007/s00018-018-2943-4Akbari E, Asemi Z, Daneshvar Kakhaki R et al (2016) Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 8:256. https://doi.10.3389/fnagi.2016.00256
Auth MK, Vora R, Farrelly P, Baillie C (2012) Childhood constipation. BMJ. 345:e7309. https://doi.10.1136/bmj.e7309
Bermudez BEBV, de Oliveira CM, de Lima Cat MN, Magdalena NIR, Celli A (2019) Gastrointestinal disorders in Down syndrome. Am J Med Genet A. 179(8):1426-1431. https://doi.10.1002/ajmg.a.61258
Bokulich NA, Chung J, Battaglia T et al (2016) Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 8(343):343ra82. https://doi.10.1126/scitranslmed.aad7121
Bonaz B, Bazin T, Pellissier S (2018) The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 12:49. https://doi.10.3389/fnins.2018.00049
Cao X, Lin P, Jiang P, Li C (2013) Characteristics of the gastrointestinal microbiome in children with autism spectrum disorder: a systematic review. Shanghai Arch Psychiatry. 25(6):342-53. https://doi.10.3969/j.issn.1002-0829.2013.06.003
Deering KE, Devine A, O’Sullivan TA, Lo J, Boyce MC, Christophersen CT (2019) Characterizing the composition of the pediatric gut microbiome: a systematic review. Nutrients. 12(1):16. https://doi.10.3390/nu12010016
Defaye M, Gervason S, Altier C (2019) Microbiota: a novel regulator of pain. J Neural Transm (Vienna). 127(4):445-465. https://doi.10.1007/s00702-019-02083-z
Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F (2024) Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med. 19(2):275-293. https://doi.10.1007/s11739-023-03374-w
Gebrayel P, Nicco C, Al Khodor S et al (2022) Microbiota medicine: towards clinical revolution. J Transl Med. 20(1):111. https://doi.10.1186/s12967-022-03296-9
Gomes DOVS, Morais MB (2019) Gut microbiota and the use of probiotics in constipation in children and adolescents: Systematic review. Rev Paul Pediatr. 38:e2018123. https://doi.10.1590/1984-0462/2020/38/2018123
Guidetti C, Salvini E, Viri M et al (2022) Randomized double-blind crossover study for evaluating a probiotic mixture on gastrointestinal and behavioral symptoms of autistic children. J Clin Med. 11(18):5263. https://doi.10.3390/jcm11185263
Ihekweazu FD, Versalovic J (2018) Development of the pediatric gut microbiome: impact on health and disease. Am J Med Sci. 356(5):413–423. https://doi.10.1016/j.amjms.2018.08.005
Kang DW, Adams JB, Gregory AC et al (2017) Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 5(1):10. https://doi.10.1186/s40168-016-0225-7
Kinashi Y, Hase K (2021) Partners in leaky gut syndrome: intestinal dysbiosis and autoimmunity. Front Immunol. 12:673708. https://doi.10.3389/fimmu.2021.673708
Kwiatkowska M, Krogulska A (2021) The significance of the gut microbiome in children with functional constipation. Adv Clin Exp Med. 30(4):471-480. https://doi.10.17219/acem/131215
Maslen C, Hodge R, Tie K, Laugharne R, Lamb K, Shankar R (2022) Constipation in autistic people and people with learning disabilities. Br J Gen Pract. 72(720):348-351. https://doi.10.3399/bjgp22X720077
Milani C, Duranti S, Bottacini F et al (2017) The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. 81(4):e00036-17. https://doi.10.1128/MMBR.00036-17
Mukhopadhya I, Segal JP, Carding SR, Hart AL, Hold GL (2019) The gut virome: the 'missing link' between gut bacteria and host immunity? Therap Adv Gastroenterol. 12:1756284819836620. https://doi.10.1177/1756284819836620
Parracho HMRT, Gibson GR, Knott F et al (2010) A double blind, placebo-controlled, crossover designed probiotic feeding study in children diagnosed with autistic spectrum disorders. Int J Probiot Prebiot. 5:69–74
Ronan V, Yeasin R, Claud EC (2021) Childhood development and the microbiome-the intestinal microbiota in maintenance of health and development of disease during childhood development. Gastroenterology. 160(2):495-506. https://doi.10.1053/j.gastro.2020.08.065
Saeed NK, Al-Beltagi M, Bediwy AS, El-Sawaf Y, Toema O (2022) Gut microbiota in various childhood disorders: Implication and indications. World J Gastroenterol. 28(18):1875-1901. https://doi.10.3748/wjg.v28.i18.1875
Santocchi E, Guiducci L, Prosperi M et al (2020) Effects of probiotic supplementation on gastrointestinal, sensory and core symptoms in autism spectrum disorders: a randomized controlled trial. Front Psychiatry. 11:550593. https://doi.10.3389/fpsyt.2020.550593
Shaaban SY, El Gendy YG, Mehanna NS et al (2018) The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutr Neurosci. 21(9):676-681. https://doi.10.1080/1028415X.2017.1347746
Siemann JK, Muller CL, Forsberg CG et al (2017). An autism-associated serotonin transporter variant disrupts multisensory processing. Transl Psychiatry. 7:e1067. https://doi.10.1038/tp.2017.1
Silva YP, Bernardi A, Frozza RL (2020) The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne). 11:25. https://doi.10.3389/fendo.2020.00025
Sivamaruthi BS, Suganthy N, Kesika P, Chaiyasut C (2020) The role of microbiome, dietary supplements, and probiotics in autism spectrum disorder. Int J Environ Res Public Health. 17(8):2647. https://doi.10.3390/ijerph17082647
Wang Q, Yang Q, Liu X (2023) The microbiota–gut–brain axis and neurodevelopmental disorders. Protein Cell. 14(10):762–775. https://doi.10.1093/procel/pwad026
West R, Roberts E, Sichel LS, Sichel J (2013) Improvements in gastrointestinal symptoms among children with autism spectrum disorder receiving the Delpro® probiotic and immunomodulator formulation. J Prob Health. 1:102. https://doi.10.4172/2329-8901.1000102


