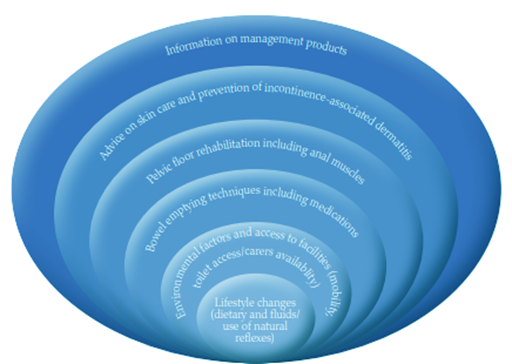
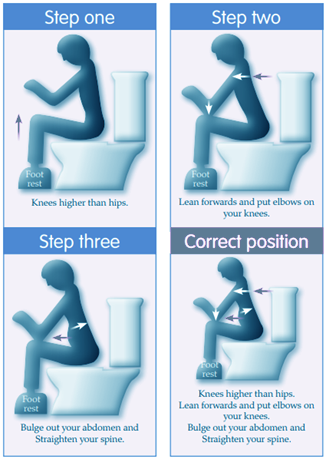
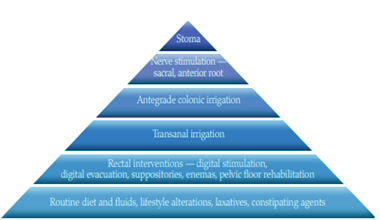
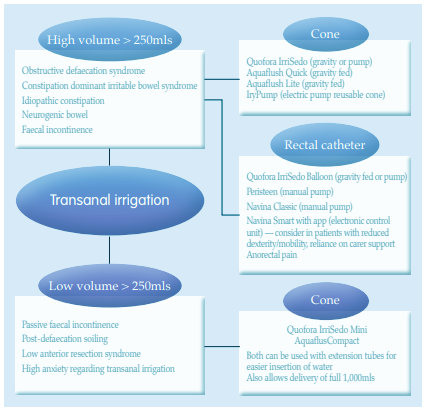
References
Assmann SL, Keszthelyi D, Kleijnen J, Anastasiou F, Bradshaw E, Brannigan AE, et al (2022) Guideline for the diagnosis and treatment of faecal incontinence — A UEG/ESCP/ESNM/ESPCG collaboration. United European Gastroenterol J 10(3): 251–86
Beeckman D, Campbell J, Campbell K, Chimentao D, Coyer F, Domansky R, et al (2015) Proceedings of the Global IAD Expert Panel. Incontinence-associated dermatitis: moving prevention forward. Available online: https://woundsinternational.com/consensus-documents/incontinence-associated-dermatitis-moving-prevention-forward/
Duelund-Jakobsen J, Worsoe J, Lundby L, Christensen P, Krough K (2016) Management of patients with faecal incontinence. Ther Adv Gastroenterol 9(1): 86–97
Emmanuel AV, Krogh K, Bazzocchi G, et al (2013) Consensus review of best practice of transanal irrigation in adults. Spinal Cord 51: 732–8
Faber W, Stolwijk-Swuste J, van Ginkel F, et al (2021) Faecal microbiota in patients with neurogenic bowel dysfunction and spinal cord injury or multiple sclerosis — a systematic review. J Clin Med 10(8): 158
Gray M, Giuliano KK (2018) Incontinence-associated dermatitis, characteristics and relationship to pressure injury: a multisite epidemiologic analysis. J Wound Ostomy Continence Nurs 45(1): 63–7
Henderson M, Tinkler L, Yiannakou Y (2018) Transanal irrigation as a treatment for bowel dysfunction. Gastrointestinal Nurs 6(4): 26–34
International Continence Society (2015) Fact Sheets. A Background to Urinary and Faecal Incontinence. Available online: www.ics.org/public
Juul T, Christensen P (2017) Prospective evaluation of transanal irrigation for faecal incontinence and constipation. Tech Coloprotol 21(5): 363–71
Leo CA, Thomas GP, Hodgkinson JD, et al (2019) The Renew R anal insert for passive faecal incontinence: a retrospective audit of our use of a novel device. Colorectal Dis 21(6): 684–8
Markland AD, Burgio KL, Whitehead WE, Richter HE, Wilcox CM, Redden DT, et al (2015) Loperamide versus psyllium fiber for treatment of fecal incontinence prescription management (FIRM) randomized clinical trial. Dis Colon Rectum 58(10): 983–93
Mekhael M, Kristensen HO, Larsen HM, et al (2021) Transanal irrigation for neurogenic bowel disease, low anterior resection syndrome, faecal incontinence and chronic constipation: a systematic review. J Clinical Med 10(4): 753
National Institute of Diabetes and Digestive and Kidney Diseases (2023) Eating, Diet and Nutrition for Fecal Incontinence. Available online: https://www.niddk.nih.gov/health-information/digestive-diseases/bowel-control-problems-fecal-incontinence/eating-diet-nutrition
Royal College of Nursing (2019) Bowel care management of lower bowel dysfunction including digital rectal examination and digital removal of faeces. Available online: www.rcn.org.uk/professional-development/publications/pub-007522
Salvatore J, Delancey Y, Igawa H, Koelbl RM, Laterza M, Serati A, et al (2017) Pathophysiology of urinary incontinence, faecal incontinence and pelvic organ prolapse in incontinence. In: Abrams P, Cardozo L, Wagg A, Wein A, eds. Incontinence. 6th edn. 6th International Consultation on Incontinence. Tokyo, September 2016: 361–496. Available online: www.epicore.ualberta.ca/Documents/PILUTS/Books/Book%20ICI%206th%20ed%202017.pdf
Scott SM, Simren M, Farmer AD, et al (2021) Chronic constipation in adults: Contemporary perspectives and clinical challenges. 1: Epidemiology, diagnosis, clinical associations, pathophysiology and investigation. Neurogastroenterol Motil 33(6): e14050
Shaw C, Wagg A (2016) Urinary and faecal incontinence in older adults. Medicine 45(1): 44–50
Yates A (2019a) Female pelvic floor 1 — anatomy and pathophysiology. Nurs Times 115(5): 18–21
Yates A (2019b) Transanal irrigation: is it the magic intervention for bowel management in individuals with bowel dysfunction? Br J Nurs 29(7): 2–6
Yates A (2020) Incontinence associated dermatitis 1: risk factors for skin damage. Nurs Times 116(3): 46–50
Yates A (2021) Faecal incontinence: a healthcare taboo. Br J Nurs 30(4): 226–8
Yates A (2023) Faecal incontinence — a forgotten symptom, part 1. J Community Nurs 37(5): 42–5
Young J (2022) Assessment and management of faecal incontinence. J Community Nurs 36(2): 51–8
Beeckman D, Campbell J, Campbell K, Chimentao D, Coyer F, Domansky R, et al (2015) Proceedings of the Global IAD Expert Panel. Incontinence-associated dermatitis: moving prevention forward. Available online: https://woundsinternational.com/consensus-documents/incontinence-associated-dermatitis-moving-prevention-forward/
Duelund-Jakobsen J, Worsoe J, Lundby L, Christensen P, Krough K (2016) Management of patients with faecal incontinence. Ther Adv Gastroenterol 9(1): 86–97
Emmanuel AV, Krogh K, Bazzocchi G, et al (2013) Consensus review of best practice of transanal irrigation in adults. Spinal Cord 51: 732–8
Faber W, Stolwijk-Swuste J, van Ginkel F, et al (2021) Faecal microbiota in patients with neurogenic bowel dysfunction and spinal cord injury or multiple sclerosis — a systematic review. J Clin Med 10(8): 158
Gray M, Giuliano KK (2018) Incontinence-associated dermatitis, characteristics and relationship to pressure injury: a multisite epidemiologic analysis. J Wound Ostomy Continence Nurs 45(1): 63–7
Henderson M, Tinkler L, Yiannakou Y (2018) Transanal irrigation as a treatment for bowel dysfunction. Gastrointestinal Nurs 6(4): 26–34
International Continence Society (2015) Fact Sheets. A Background to Urinary and Faecal Incontinence. Available online: www.ics.org/public
Juul T, Christensen P (2017) Prospective evaluation of transanal irrigation for faecal incontinence and constipation. Tech Coloprotol 21(5): 363–71
Leo CA, Thomas GP, Hodgkinson JD, et al (2019) The Renew R anal insert for passive faecal incontinence: a retrospective audit of our use of a novel device. Colorectal Dis 21(6): 684–8
Markland AD, Burgio KL, Whitehead WE, Richter HE, Wilcox CM, Redden DT, et al (2015) Loperamide versus psyllium fiber for treatment of fecal incontinence prescription management (FIRM) randomized clinical trial. Dis Colon Rectum 58(10): 983–93
Mekhael M, Kristensen HO, Larsen HM, et al (2021) Transanal irrigation for neurogenic bowel disease, low anterior resection syndrome, faecal incontinence and chronic constipation: a systematic review. J Clinical Med 10(4): 753
National Institute of Diabetes and Digestive and Kidney Diseases (2023) Eating, Diet and Nutrition for Fecal Incontinence. Available online: https://www.niddk.nih.gov/health-information/digestive-diseases/bowel-control-problems-fecal-incontinence/eating-diet-nutrition
Royal College of Nursing (2019) Bowel care management of lower bowel dysfunction including digital rectal examination and digital removal of faeces. Available online: www.rcn.org.uk/professional-development/publications/pub-007522
Salvatore J, Delancey Y, Igawa H, Koelbl RM, Laterza M, Serati A, et al (2017) Pathophysiology of urinary incontinence, faecal incontinence and pelvic organ prolapse in incontinence. In: Abrams P, Cardozo L, Wagg A, Wein A, eds. Incontinence. 6th edn. 6th International Consultation on Incontinence. Tokyo, September 2016: 361–496. Available online: www.epicore.ualberta.ca/Documents/PILUTS/Books/Book%20ICI%206th%20ed%202017.pdf
Scott SM, Simren M, Farmer AD, et al (2021) Chronic constipation in adults: Contemporary perspectives and clinical challenges. 1: Epidemiology, diagnosis, clinical associations, pathophysiology and investigation. Neurogastroenterol Motil 33(6): e14050
Shaw C, Wagg A (2016) Urinary and faecal incontinence in older adults. Medicine 45(1): 44–50
Yates A (2019a) Female pelvic floor 1 — anatomy and pathophysiology. Nurs Times 115(5): 18–21
Yates A (2019b) Transanal irrigation: is it the magic intervention for bowel management in individuals with bowel dysfunction? Br J Nurs 29(7): 2–6
Yates A (2020) Incontinence associated dermatitis 1: risk factors for skin damage. Nurs Times 116(3): 46–50
Yates A (2021) Faecal incontinence: a healthcare taboo. Br J Nurs 30(4): 226–8
Yates A (2023) Faecal incontinence — a forgotten symptom, part 1. J Community Nurs 37(5): 42–5
Young J (2022) Assessment and management of faecal incontinence. J Community Nurs 36(2): 51–8



.png)