References
Abernathy J, Guy R, Sheridan EA, et al (2017) Epidemiology of Escherichia coli bacteraemia in England, Results of an enhanced sentinel surveillance programme. J Hosp Infect 95(4): 365–75
Beattie M, Taylor J (2011) Silver alloy versus uncoated urinary catheters: a systematic review of the literature. J Clin Nurs 20: 2098–2108
Chang R, Todd GM, Chenoweth CE (2011) Epidemiology of hospital acquired urinary tract related bloodstream infection at a university hospital. Infect Contr Hosp Epidemiol 32(11): 1127–9
Davey G (2015) Troubleshooting indwelling catheter problems in the community. J Community Nurs 29(4): 67–74
Elvy J, Colville A (2009) Catheter associated urinary tract infection: what is it, what causes it, and how can we prevent it? J Infect Prevent 10(2): 36–41
Feneley R, Hopley I, Wells P (2015) Urinary catheters history: current status, adverse events and research agenda. J Med Eng Technol 39(8): 459–70
Garcia MM, Gulati S, Liepmann D, et al (2007) Traditional Foley drainage systems — do they drain the bladder? J Urol 177: 203–7
Geng V, Cobussen-Boekhorst H, Farrell J (2012) Catheterisation: Indwelling in Adults. European Association of Urology Nurses (EAUN), Holland
Gibney LE (2016) Blocked urinary catheters: can they be better managed? Br J Nurs 25(15): 828-33
Hanchett M (2002) Techniques for stabilising urinary catheters: tape may be the oldest method but it’s not the only one. Am J Nurs 102(3): 44–48
Hayes W (2009) Looking past the silver lining. Materials Management in Healthcare January: 20–23. Available online: matmanmag.com
Healthcare Infection Control Practices Advisory Committee (2009) Guideline for Prevention of Catheter-associated Urinary Tract Infection. Available online: www.cdc.gov/infectioncontrol/pdf/ guidelines/cauti-guidelines.pdf (accessed 1 February, 2018)
Health Protection Scotland (2012) Scottish National Point Prevalence Survey of Healthcare Associated Infection and Antimicrobial Prescribing 2011. Available online: http://bit.ly/1SJCIcK
Health and Safety Executive (2011) Natural rubber latex sensitisation in health and social care. Available online: www.hse.gov. uk/foi/internalops/sims/pub_serv/071106. htm (accessed 30 January, 2018)
Holroyd S (2016) Innovation in catheter securement devices: minimising risk of infection, trauma and pain. Br J Community Nurs 21(5): 256–9
Kyle G (2009) Lubricating gels in urethral catheterisation — the evidence. Continence UK 3(1): 35–7
Leaver R (2017) Understanding long-term catheterisation for effective bladder drainage. J Community Nurs 31(5): 43–8
Levers H (2014) Switching to an antimicrobial solution for skin cleansing before urinary catheterisation. Br J Community Nurs 19(2): 66–71
Loveday HP, Wilson JA, Pratt RJ (2014) EPIC 3: national evidence-based guidelines for preventing healthcare associated infections in NHS hospitals in England. J Hosp Infect 86(Suppl 1): S1–S70
Marinho S, Oliver P, Hughes D (2013) Chlorhexidine Allergy Policy: Screening and Management of Patients with Chlorhexidine Allergy. University Hospital of South Manchester NHS Foundation Trust, Manchester
National Institute for Health and Care Excellence (2014) Long Term Urinary Catheters: Prevention and Control of Healthcare-associated Infections in Primary and Community Care. NICE, London
National Institute for Health and Care Excellence (2017) Healthcare-associated Infections: Prevention and Control in Primary and Community Care. Clinical guideline 139. NICE, London
National Patient Safety Agency (2009) Rapid Response Report: minimising risks of suprapubic catheter insertion (adults only). Available online: www.nrls.npsa.nhs.uk/ alerts/?entryid45=61917 (accessed 2 February, 2018)
NHS Improvement (2017) Preventing Healthcare-associated Gram-negative Bloodstream Infections: An Improvement Resource. Public Health England, London
Pannek J, Vesweber A (2011) Clinical utility of an antimicrobial blocking solution in patients with an indwelling catheter. Aktuelle Urol 42: 51–4
Pellowe C (2009) EPIC 2 infection prevention guidelines — best practice. Continence UK 3(3): 28–33
Pickard R, Lam T, MacLennan GL (2012) Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet 380(9857): 1927–35
Ramakrishnan K, Mold J (2004) Urinary catheters: a review. Int J Fam Pract. Available online: http://ispub.com/ IJFP/3/2/4596
Rowley S, Clare S, Macqueen S, Molyneux R (2010) ANTT v2: an updated practice framework for aseptic technique. Br J Nurs 19(5 Suppl): S5–11
Royal College of Nursing (2012) Catheter Care: RCN Guidance for Nurses. RCN, London Royal College of Physicians (2005) Report of the National Audit of Continence Care in Older People (65 years and above) in England, Wales and Northern Ireland. Royal College of Physicians, London
Sandle T (2013) Using an antimicrobial skin cleanser before catheterisation. J Community Nurs 27(5): 30–4
Simpson P (2017) Long-term urethral catheterisation: guidelines for community nurses. Br J Nurs 26(9 Suppl): S22–S26
Sperling H, Eisenhardt A, Mumperow E (2014) Review of the use of triclosan in permanent catheters. Der Urologe 53: 1512–17
Spinks J (2013) Urinary incontinence and the importance of catheter fixation. J Community Nurs 27(5 Suppl): S24–S29
Stickler D, Young R, Jones G, Sabbuba N, Morris N (2003) Why are foley catheters so vulnerable to encrustation and blockage by crystalline bacterial biofilm? Urol Res 31: 306–11
Stickler DJ, Feneley RCL (2010) The encrustation and blockage of longterm indwelling bladder catheters: a way forward in prevention and control. Spinal Cord 48(11): 784–90
Tenke P, Kovacs B, Bjerklund Johansen TE (2008) European and Asian guidelines on management and prevention of catheter-associated urinary tract infections. Int J Antimicrob Agents 31(1 Suppl): S68–78
Turner B, Dickens N (2011) Long-term urethral catheterisation. Nurs Standard 25(24): 49–55
Tzortzis V, Gravas S, Melekos MM, de la Rosette JJ (2009) Intraurethral lubricants: a critical literature review and recommendations. J Endourol 23(5): 821–6
Williams C (2017) Making a choice of catheterisation gel and the role of chlorhexidine. Br J Community Nurs 22(7): 346–51
Wilson M (2016) Urinary catheterisation in the community: exploring challenges and solutions. Br J Community Nurs 21(10): 443
Woodward S (2014) Securing urethral catheters can help to reduce their complications. Br J Neuro Nurs 10(4): 162–5
Wound, Ostomy and Continence Nurses Society (2012) Indwelling Urinary Catheter Securement: Best Practice for Clinicians. WOCN, USA
Yarde D (2015) Managing indwelling urinary catheters in adults. Nurs Times 111(22): 12–13
Yates A (2016) Indwelling urinary catheterisation: what is best practice? Br J Nurs 25(9 Suppl): S4–12
Beattie M, Taylor J (2011) Silver alloy versus uncoated urinary catheters: a systematic review of the literature. J Clin Nurs 20: 2098–2108
Chang R, Todd GM, Chenoweth CE (2011) Epidemiology of hospital acquired urinary tract related bloodstream infection at a university hospital. Infect Contr Hosp Epidemiol 32(11): 1127–9
Davey G (2015) Troubleshooting indwelling catheter problems in the community. J Community Nurs 29(4): 67–74
Elvy J, Colville A (2009) Catheter associated urinary tract infection: what is it, what causes it, and how can we prevent it? J Infect Prevent 10(2): 36–41
Feneley R, Hopley I, Wells P (2015) Urinary catheters history: current status, adverse events and research agenda. J Med Eng Technol 39(8): 459–70
Garcia MM, Gulati S, Liepmann D, et al (2007) Traditional Foley drainage systems — do they drain the bladder? J Urol 177: 203–7
Geng V, Cobussen-Boekhorst H, Farrell J (2012) Catheterisation: Indwelling in Adults. European Association of Urology Nurses (EAUN), Holland
Gibney LE (2016) Blocked urinary catheters: can they be better managed? Br J Nurs 25(15): 828-33
Hanchett M (2002) Techniques for stabilising urinary catheters: tape may be the oldest method but it’s not the only one. Am J Nurs 102(3): 44–48
Hayes W (2009) Looking past the silver lining. Materials Management in Healthcare January: 20–23. Available online: matmanmag.com
Healthcare Infection Control Practices Advisory Committee (2009) Guideline for Prevention of Catheter-associated Urinary Tract Infection. Available online: www.cdc.gov/infectioncontrol/pdf/ guidelines/cauti-guidelines.pdf (accessed 1 February, 2018)
Health Protection Scotland (2012) Scottish National Point Prevalence Survey of Healthcare Associated Infection and Antimicrobial Prescribing 2011. Available online: http://bit.ly/1SJCIcK
Health and Safety Executive (2011) Natural rubber latex sensitisation in health and social care. Available online: www.hse.gov. uk/foi/internalops/sims/pub_serv/071106. htm (accessed 30 January, 2018)
Holroyd S (2016) Innovation in catheter securement devices: minimising risk of infection, trauma and pain. Br J Community Nurs 21(5): 256–9
Kyle G (2009) Lubricating gels in urethral catheterisation — the evidence. Continence UK 3(1): 35–7
Leaver R (2017) Understanding long-term catheterisation for effective bladder drainage. J Community Nurs 31(5): 43–8
Levers H (2014) Switching to an antimicrobial solution for skin cleansing before urinary catheterisation. Br J Community Nurs 19(2): 66–71
Loveday HP, Wilson JA, Pratt RJ (2014) EPIC 3: national evidence-based guidelines for preventing healthcare associated infections in NHS hospitals in England. J Hosp Infect 86(Suppl 1): S1–S70
Marinho S, Oliver P, Hughes D (2013) Chlorhexidine Allergy Policy: Screening and Management of Patients with Chlorhexidine Allergy. University Hospital of South Manchester NHS Foundation Trust, Manchester
National Institute for Health and Care Excellence (2014) Long Term Urinary Catheters: Prevention and Control of Healthcare-associated Infections in Primary and Community Care. NICE, London
National Institute for Health and Care Excellence (2017) Healthcare-associated Infections: Prevention and Control in Primary and Community Care. Clinical guideline 139. NICE, London
National Patient Safety Agency (2009) Rapid Response Report: minimising risks of suprapubic catheter insertion (adults only). Available online: www.nrls.npsa.nhs.uk/ alerts/?entryid45=61917 (accessed 2 February, 2018)
NHS Improvement (2017) Preventing Healthcare-associated Gram-negative Bloodstream Infections: An Improvement Resource. Public Health England, London
Pannek J, Vesweber A (2011) Clinical utility of an antimicrobial blocking solution in patients with an indwelling catheter. Aktuelle Urol 42: 51–4
Pellowe C (2009) EPIC 2 infection prevention guidelines — best practice. Continence UK 3(3): 28–33
Pickard R, Lam T, MacLennan GL (2012) Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet 380(9857): 1927–35
Ramakrishnan K, Mold J (2004) Urinary catheters: a review. Int J Fam Pract. Available online: http://ispub.com/ IJFP/3/2/4596
Rowley S, Clare S, Macqueen S, Molyneux R (2010) ANTT v2: an updated practice framework for aseptic technique. Br J Nurs 19(5 Suppl): S5–11
Royal College of Nursing (2012) Catheter Care: RCN Guidance for Nurses. RCN, London Royal College of Physicians (2005) Report of the National Audit of Continence Care in Older People (65 years and above) in England, Wales and Northern Ireland. Royal College of Physicians, London
Sandle T (2013) Using an antimicrobial skin cleanser before catheterisation. J Community Nurs 27(5): 30–4
Simpson P (2017) Long-term urethral catheterisation: guidelines for community nurses. Br J Nurs 26(9 Suppl): S22–S26
Sperling H, Eisenhardt A, Mumperow E (2014) Review of the use of triclosan in permanent catheters. Der Urologe 53: 1512–17
Spinks J (2013) Urinary incontinence and the importance of catheter fixation. J Community Nurs 27(5 Suppl): S24–S29
Stickler D, Young R, Jones G, Sabbuba N, Morris N (2003) Why are foley catheters so vulnerable to encrustation and blockage by crystalline bacterial biofilm? Urol Res 31: 306–11
Stickler DJ, Feneley RCL (2010) The encrustation and blockage of longterm indwelling bladder catheters: a way forward in prevention and control. Spinal Cord 48(11): 784–90
Tenke P, Kovacs B, Bjerklund Johansen TE (2008) European and Asian guidelines on management and prevention of catheter-associated urinary tract infections. Int J Antimicrob Agents 31(1 Suppl): S68–78
Turner B, Dickens N (2011) Long-term urethral catheterisation. Nurs Standard 25(24): 49–55
Tzortzis V, Gravas S, Melekos MM, de la Rosette JJ (2009) Intraurethral lubricants: a critical literature review and recommendations. J Endourol 23(5): 821–6
Williams C (2017) Making a choice of catheterisation gel and the role of chlorhexidine. Br J Community Nurs 22(7): 346–51
Wilson M (2016) Urinary catheterisation in the community: exploring challenges and solutions. Br J Community Nurs 21(10): 443
Woodward S (2014) Securing urethral catheters can help to reduce their complications. Br J Neuro Nurs 10(4): 162–5
Wound, Ostomy and Continence Nurses Society (2012) Indwelling Urinary Catheter Securement: Best Practice for Clinicians. WOCN, USA
Yarde D (2015) Managing indwelling urinary catheters in adults. Nurs Times 111(22): 12–13
Yates A (2016) Indwelling urinary catheterisation: what is best practice? Br J Nurs 25(9 Suppl): S4–12



 Silicone catheters have a wider internal lumen due to the composition, i.e. thinner walls than latex or coated catheters, and those made of 100% silicone are hypoallergenic for the majority of the population (Loveday et al, 2014). Silicone is slightly more rigid than latex and has a tendency to cause ‘cuffing’ or ‘ridging’ around the deflated balloon, which may in turn become attached to the urethral or suprapubic tract on removal, causing trauma (Geng et al, 2012; Feneley et al, 2015). In the author’s experience, this issue can be managed by completely deflating the catheter balloon and then inserting 1ml of sterile water to smooth out the edges of the balloon, which helps prevent the catheter from sticking to the urethral or abdominal tract tissues on removal. This, in turn, will reduce potential trauma.
Silicone catheters have a wider internal lumen due to the composition, i.e. thinner walls than latex or coated catheters, and those made of 100% silicone are hypoallergenic for the majority of the population (Loveday et al, 2014). Silicone is slightly more rigid than latex and has a tendency to cause ‘cuffing’ or ‘ridging’ around the deflated balloon, which may in turn become attached to the urethral or suprapubic tract on removal, causing trauma (Geng et al, 2012; Feneley et al, 2015). In the author’s experience, this issue can be managed by completely deflating the catheter balloon and then inserting 1ml of sterile water to smooth out the edges of the balloon, which helps prevent the catheter from sticking to the urethral or abdominal tract tissues on removal. This, in turn, will reduce potential trauma. Silver-coated catheters were introduced in the 1980s and are manufactured either as silicone, hydrogel or latex catheters with a thin layer silver alloy coating. Silver has long been recognised for its natural antimicrobial properties (Hayes, 2009), and these catheters were initially thought to significantly reduce the incidence of catheterassociated urinary tract infections (CAUTIs) (Pellowe, 2009; Stickler and Feneley, 2010; Pickard et al, 2012). However, it has since been acknowledged that the antimicrobial effect is short-lived and lasts no longer than 28 days (Tenke et al, 2008; Hayes, 2009; Beattie and Taylor, 2011; Geng et al, 2012; Loveday et al, 2014; Feneley et al, 2015). This means that the catheter has no added benefit in reducing infections after the 28 days and, therefore, would need changing to ensure optimum antimicrobial effect is continued. This will have cost implications, as these types of catheters are more expensive.
Silver-coated catheters were introduced in the 1980s and are manufactured either as silicone, hydrogel or latex catheters with a thin layer silver alloy coating. Silver has long been recognised for its natural antimicrobial properties (Hayes, 2009), and these catheters were initially thought to significantly reduce the incidence of catheterassociated urinary tract infections (CAUTIs) (Pellowe, 2009; Stickler and Feneley, 2010; Pickard et al, 2012). However, it has since been acknowledged that the antimicrobial effect is short-lived and lasts no longer than 28 days (Tenke et al, 2008; Hayes, 2009; Beattie and Taylor, 2011; Geng et al, 2012; Loveday et al, 2014; Feneley et al, 2015). This means that the catheter has no added benefit in reducing infections after the 28 days and, therefore, would need changing to ensure optimum antimicrobial effect is continued. This will have cost implications, as these types of catheters are more expensive.
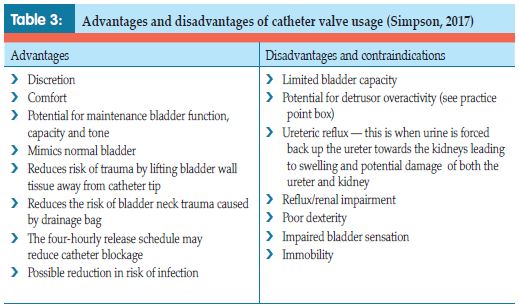
 The balloon size of the catheter needs to be considered when choosing a product. Manufacturers produce Foley catheters with balloon sizes ranging from 5mls to 30mls. The larger balloons can increase the trauma and infection risk at the bladder neck, sphincter or suprapubic entry site, and leave a higher residual volume of urine within the bladder, thereby increasing the risk of infection from static urine (Garcia et al, 2007; Feneley et al, 2015). Larger balloons may also increase the incidence of urine bypassing, bladder spasms, pain and discomfort (Simpson, 2017). Larger balloon size catheters are commonest in urology when the Ch size is much larger, and the catheter is three-way rather than the standard two-way product to enable irrigation and effective drainage postoperatively. As the larger balloon size can cause trauma and increase the risk of infection, their use should be short term. A clinical indication for the choice of catheter should always be documented and the least risky option used.
The balloon size of the catheter needs to be considered when choosing a product. Manufacturers produce Foley catheters with balloon sizes ranging from 5mls to 30mls. The larger balloons can increase the trauma and infection risk at the bladder neck, sphincter or suprapubic entry site, and leave a higher residual volume of urine within the bladder, thereby increasing the risk of infection from static urine (Garcia et al, 2007; Feneley et al, 2015). Larger balloons may also increase the incidence of urine bypassing, bladder spasms, pain and discomfort (Simpson, 2017). Larger balloon size catheters are commonest in urology when the Ch size is much larger, and the catheter is three-way rather than the standard two-way product to enable irrigation and effective drainage postoperatively. As the larger balloon size can cause trauma and increase the risk of infection, their use should be short term. A clinical indication for the choice of catheter should always be documented and the least risky option used.