References
Booth F, Clarkson M (2012) Principles of catheterisation. J Community Nurs 26(3): 37–41
Bladder & Bowel Community (2018) Bladder. Available online: www. bladderandbowelfoundation.org/bladder
British Association of Urological Surgeons (2017) Urinary infection (adult). Available online: www.baus.org.uk.
Buckley BS, Lapitan MCM (2009) Prevalence of urinary and faecal incontinence and nocturnal enuresis and attitudes to treatment and help seeking amongst a community-based representative sample of adults in the United Kingdom. Int J Clin Practice 2009; 63(4): 568–73
Centers for Disease Control and Prevention (2009) Guideline for prevention of catheter-associated urinary tract infections. Available online: www. cdc.gov/infectioncontrol/guidelines/cauti/index.html
Centers for Disease Control and Prevention (2014) Infection control. Available online: www.cdc.gov/ infectioncontrol/guidelines/index.html
Centres for Disease Control and Prevention (2015) Healthcare-associated Infections: Catheter-associated Urinary Tract Infection (CAUTI). Available online at: www.cdc.gov.
Davey G (2015) Troubleshooting indwelling catheter problems in the community. J Community Nurs 29(4): 67–74
European Centre for Disease Control and Prevention (2013) Point prevalence survey of healthcare associated infections and antimicrobial use in European longterm care facilities. Available online: https://ecdc.europa.eu/en/publicationsdata/point-prevalence-surveyhealthcare-associated-infections-andantimicrobial-use-2
Fisher E, Dahm P, Omar IM (2017) Challenges of catheter associated urinary tract infection: is prevention better than cure? Urol News 22(1). Available online at www.urologynews. uk.com.
Health Economics Research Unit (HERU) (2015) Reducing hospital infections: which catheter? Available online: www.abdn. ac.uk/heru/news/8609/
Loveday HP, Wilson JA, Pratt RJ, et al (2014) epic 3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 86(Suppl 1): S1–S70
Meddings J, Saint S, Fowler KE, et al (2015) The Ann Arbor criteria for appropriate urinary catheter use in hospitalized patients: Results obtained by using the RAND/UCLA Appropriateness Method. Ann Intern Med 162(Suppl 9): S1–34
National Institute for Health and Care Excellence (2010) Constipation in children and young people: diagnosis and management. Clinical guideline [CG 99]. NICE, London. Available online: www. nice.org.uk/guidance/cg99
National Institute for Health and Care Excellence (2012) Healthcare-associated infections: prevention and control in primary can community care. NICE, London. Available online: www.nice.org.uk/guidance/cg139/ resources/healthcareassociatedinfections- prevention-and-controlin- primary-and-community-carepdf-35109518767045
National Institute for Health and Care Excellence (2013) Urinary incontinence: the management of urinary incontinence in women. Clinical guideline [CG] 171. Updated November 2015. NICE, London. Available online: www.nice. org.uk/guidance/cg171
NHS England (2015) Excellence in continence care: Practical guidance for commissioners, providers, health and social care staff and information for the public. NHS England, London
Nicolle L (2014) Catheter associated urinary tract infections. Antimicrobial Resist Infection Control 3: 23
Nursing and Midwifery Council (NMC) (2015) The Code: Professional Standards of Practice and Behaviours for Nurse and Midwives. NMC, London
Richards D, Toop L, Chambers S, Fletcher L (2005) Response to antibiotics of women with urinary tract infection but negative dipstick urine test results: double blind randomised controlled trial. BMJ 331(7509): 143
Sørbye LW, Finne-Soveri H, Ljunggren G, et al (2005) Indwelling catheter use in home care: elderly, aged 65+, in 11 different countries in Europe. Age Ageing 34(4): 377–81
Scottish Intercollegiate Guidelines Network (2012) Management of suspected bacterial urinary tract infection in adults. A national clinical guideline. SIGN, Edinburgh. Available online: www.sign.ac.uk/assets/sign88.pdf
Bladder & Bowel Community (2018) Bladder. Available online: www. bladderandbowelfoundation.org/bladder
British Association of Urological Surgeons (2017) Urinary infection (adult). Available online: www.baus.org.uk.
Buckley BS, Lapitan MCM (2009) Prevalence of urinary and faecal incontinence and nocturnal enuresis and attitudes to treatment and help seeking amongst a community-based representative sample of adults in the United Kingdom. Int J Clin Practice 2009; 63(4): 568–73
Centers for Disease Control and Prevention (2009) Guideline for prevention of catheter-associated urinary tract infections. Available online: www. cdc.gov/infectioncontrol/guidelines/cauti/index.html
Centers for Disease Control and Prevention (2014) Infection control. Available online: www.cdc.gov/ infectioncontrol/guidelines/index.html
Centres for Disease Control and Prevention (2015) Healthcare-associated Infections: Catheter-associated Urinary Tract Infection (CAUTI). Available online at: www.cdc.gov.
Davey G (2015) Troubleshooting indwelling catheter problems in the community. J Community Nurs 29(4): 67–74
European Centre for Disease Control and Prevention (2013) Point prevalence survey of healthcare associated infections and antimicrobial use in European longterm care facilities. Available online: https://ecdc.europa.eu/en/publicationsdata/point-prevalence-surveyhealthcare-associated-infections-andantimicrobial-use-2
Fisher E, Dahm P, Omar IM (2017) Challenges of catheter associated urinary tract infection: is prevention better than cure? Urol News 22(1). Available online at www.urologynews. uk.com.
Health Economics Research Unit (HERU) (2015) Reducing hospital infections: which catheter? Available online: www.abdn. ac.uk/heru/news/8609/
Loveday HP, Wilson JA, Pratt RJ, et al (2014) epic 3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 86(Suppl 1): S1–S70
Meddings J, Saint S, Fowler KE, et al (2015) The Ann Arbor criteria for appropriate urinary catheter use in hospitalized patients: Results obtained by using the RAND/UCLA Appropriateness Method. Ann Intern Med 162(Suppl 9): S1–34
National Institute for Health and Care Excellence (2010) Constipation in children and young people: diagnosis and management. Clinical guideline [CG 99]. NICE, London. Available online: www. nice.org.uk/guidance/cg99
National Institute for Health and Care Excellence (2012) Healthcare-associated infections: prevention and control in primary can community care. NICE, London. Available online: www.nice.org.uk/guidance/cg139/ resources/healthcareassociatedinfections- prevention-and-controlin- primary-and-community-carepdf-35109518767045
National Institute for Health and Care Excellence (2013) Urinary incontinence: the management of urinary incontinence in women. Clinical guideline [CG] 171. Updated November 2015. NICE, London. Available online: www.nice. org.uk/guidance/cg171
NHS England (2015) Excellence in continence care: Practical guidance for commissioners, providers, health and social care staff and information for the public. NHS England, London
Nicolle L (2014) Catheter associated urinary tract infections. Antimicrobial Resist Infection Control 3: 23
Nursing and Midwifery Council (NMC) (2015) The Code: Professional Standards of Practice and Behaviours for Nurse and Midwives. NMC, London
Richards D, Toop L, Chambers S, Fletcher L (2005) Response to antibiotics of women with urinary tract infection but negative dipstick urine test results: double blind randomised controlled trial. BMJ 331(7509): 143
Sørbye LW, Finne-Soveri H, Ljunggren G, et al (2005) Indwelling catheter use in home care: elderly, aged 65+, in 11 different countries in Europe. Age Ageing 34(4): 377–81
Scottish Intercollegiate Guidelines Network (2012) Management of suspected bacterial urinary tract infection in adults. A national clinical guideline. SIGN, Edinburgh. Available online: www.sign.ac.uk/assets/sign88.pdf



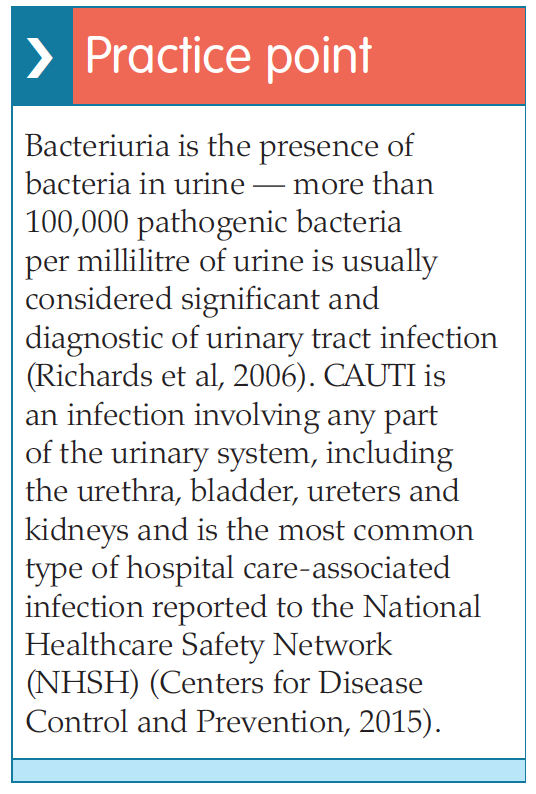 While catheterisation is a common procedure undertaken by healthcare professionals, it is neither simple nor risk-free (Booth and Clarkson, 2012).
While catheterisation is a common procedure undertaken by healthcare professionals, it is neither simple nor risk-free (Booth and Clarkson, 2012).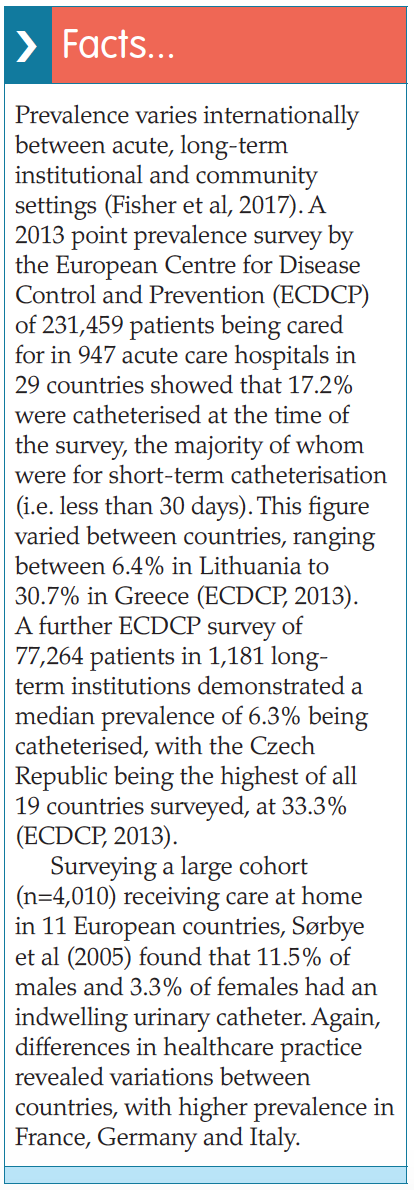 Progression from bacteriuria to CAUTI causes both increased risk to the patient and healthcare costs, i.e. longer hospital stays, treatment, etc (Loveday et al, 2014). Risk factors for progression to CAUTI include:
Progression from bacteriuria to CAUTI causes both increased risk to the patient and healthcare costs, i.e. longer hospital stays, treatment, etc (Loveday et al, 2014). Risk factors for progression to CAUTI include:
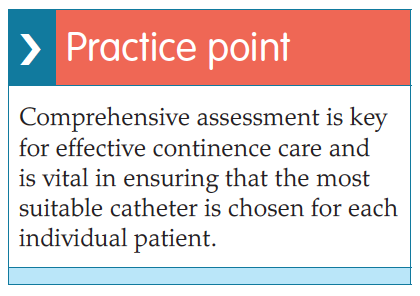 According to NHS England (2015), regular audits by the Healthcare Quality Improvement Partnership (HQIP), the latest being in 2010, show that despite the amount of guidance available, the quality of continence care remains variable across the country and poorer overall for the elderly. In the author’s clinical experience, many continence problems can be cured and certainly managed better.
According to NHS England (2015), regular audits by the Healthcare Quality Improvement Partnership (HQIP), the latest being in 2010, show that despite the amount of guidance available, the quality of continence care remains variable across the country and poorer overall for the elderly. In the author’s clinical experience, many continence problems can be cured and certainly managed better.
 Urinary catheters can sometimes fall out. If this occurs, patients should contact their district or specialist urology nurse immediately so that it can be replaced. If this continues to happen, patients may be referred to the urologist for further advice and reassessment of the type of catheter in use.
Urinary catheters can sometimes fall out. If this occurs, patients should contact their district or specialist urology nurse immediately so that it can be replaced. If this continues to happen, patients may be referred to the urologist for further advice and reassessment of the type of catheter in use.
 In the UK, there are over 14 million adults who have bladder control problems and six and a half million with bowel control problems (Buckley and Lapitan, 2009). In addition, 900,000 children and young people suffer from bladder and bowel dysfunction (NICE, 2010).
In the UK, there are over 14 million adults who have bladder control problems and six and a half million with bowel control problems (Buckley and Lapitan, 2009). In addition, 900,000 children and young people suffer from bladder and bowel dysfunction (NICE, 2010).